Class 4 Medicines Defect Information: Novartis Pharmaceuticals, Simulect 10mg & 20mg powder and solvent for Solution for injection or infusion, EL (23)A/17
Novartis Pharmaceuticals has informed the MHRA that the solvent (water for injections in ampoules) co-packed with the impacted batches of Simulect powder for injection, may contain glass fragments approximately 20 – 800 µm in size.
MDR number
MDR 070-04/23
Company name
Novartis Pharmaceuticals
Product name
Simulect 10mg powder and solvent for Solution for injection or infusion
SNOMED Code
5007811000001106
| Batch No | Expiry Date | Pack Size | First Distributed |
|---|---|---|---|
| SHVF3 | 30/09/2025 | 1 x 10mg glass vial, 1 x WFI ampoule | 28/02/2023 |
Active Pharmaceutical Ingredient: Basiliximab
Product name
Simulect 20mg powder and solvent for Solution for injection or infusion
SNOMED Code
4773411000001103
| Batch No | Expiry Date | Pack Size | First Distributed |
|---|---|---|---|
| SHWU5 | 30/09/2025 | 1 x 20mg glass vial, 1 x WFI ampoule | 21/03/2023 |
| SHEW5 | 31/07/2025 | 1 x 20mg glass vial, 1 x WFI ampoule | 08/12/2022 |
| SHFV1 | 31/07/2025 | 1 x 20mg glass vial, 1 x WFI ampoule | 22/12/2022 |
Active Pharmaceutical Ingredient: Basiliximab
Brief description of the problem
Novartis Pharmaceuticals has informed the MHRA that the solvent (water for injections in ampoules) co-packed with the impacted batches of Simulect powder for injection, may contain glass fragments approximately 20 – 800 µm in size. Therefore, the included solvent should not be used, but replaced with an alternative water for injection. The quality of the Simulect vials themselves is not affected and due to supply considerations, the impacted batches are not being recalled.
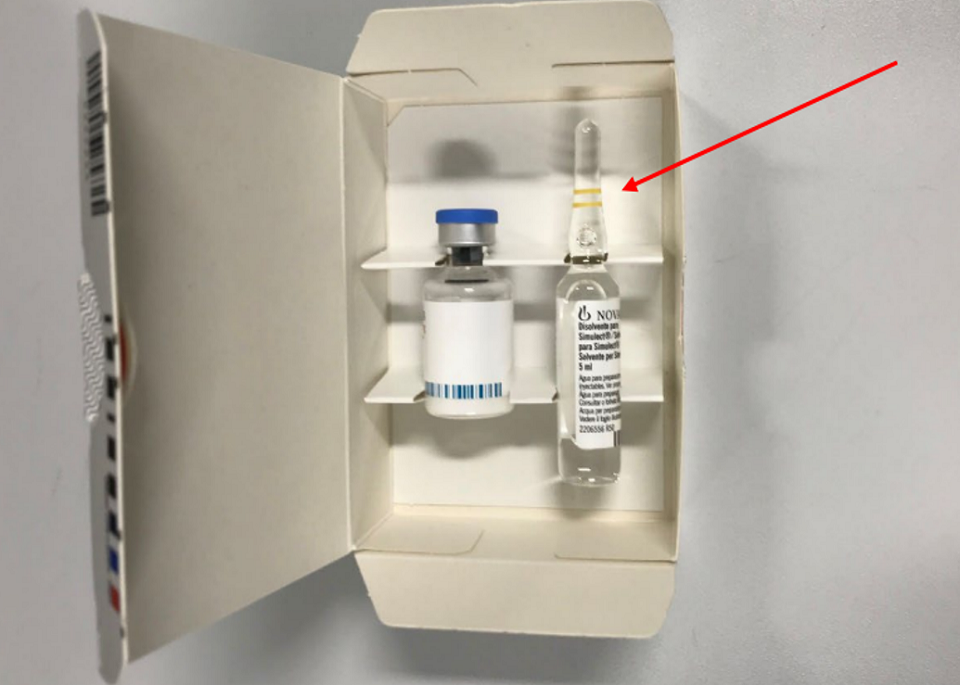
The solvent (water for injection in ampoules) shown below in Figure 1 should be discarded as per the advice for healthcare professionals below.
Figure 1 - Solvent (water for injection) ampoule
Advice for healthcare professionals
Novartis Pharmaceuticals has directly notified customers and previously shared considerations for the impacted batches mentioned in this notification. The following actions should be taken:
- discard the solvent (water for injections) co-packed with batches of Simulect at the time of opening the pack. The entire ampoule should be discarded carefully and as per local procedures.
- source alternative water for injection for use as the solvent with Simulect powder. These options should be readily available across secondary care settings.
- the water for injection should comply with European Pharmacopoeia requirements for water for injection, which will be the case if using a licensed product.
- healthcare professionals should continue to safely administer Simulect from the impacted batches using an alternative source of water for injections, following reconstitution as per the Summary of Product Characteristics (SmPC):
- forward a copy of this notification to all facilities or departments within your hospital or clinic that may use this product.
Additionally healthcare professionals should complete the Customer Reply Form (Appendix 1 at the bottom of the PDF) and return it to Novartis by email ([email protected]) within 3 working days.
Advice for patients
No further action is required by patients. This product is administered by healthcare professionals directly. If you have concerns about a medicine you may be using, please contact your healthcare professional.
Any suspected adverse reactions should be reported via the MHRA Yellow Card scheme.
Further Information
For more information, medical or supply enquiries, please contact 01276 698370, or email [email protected]
Recipients of this Medicines Notification should bring it to the attention of relevant contacts by copy of this notice. NHS regional teams are asked to forward this to community pharmacists and dispensing general practitioners for information.
Yours faithfully
Defective Medicines Report Centre
10 South Colonnade
Canary Wharf
London
E14 4PU
Telephone +44 (0)20 3080 6574
Download documents