Propofol 10mg/ml (1%) Emulsion for Injection/Infusion 50ml presentation - incorrect colour identifying volume on package
(Uab Norameda) distributor Peckforton Pharmaceuticals Limited has identified an error on the presentation of the 50ml carton - the coloured band on the package should be purple not pink although text is correct (EL(15)A/06).
16 July 2015
Class 4 medicines defect information
Caution in use
Hospital pharmacy, operating department, ward level
MDR 45-06/15
Product details
Uab Noramedia
Propofol 10mg/ml (1%) Emulsion for Injection/Infusion 50ml Presentation
PL 32911/0004
Alert details
Peckforton Pharmaceuticals Limited, distributor of the above product, has notified us of an error on the carton of the 50ml presentation of Propofol 10mg/ml (1%) Emulsion for Injection/Infusion. The coloured band which identifies the volume and total propofol content contained in the vial should be purple. For batches released to the market since November 2014, however, the band is pink. The text within the coloured band and on the vial label is correct. See below for photographs.
To ensure continuity of supply, distribution of affected stock will continue and it will be necessary to release further batches.
For further information, please contact Peckforton Medical information on 01270 582255 or email [email protected].
Recipients of this drug alert should bring this to the attention of relevant contacts by copy of this letter.
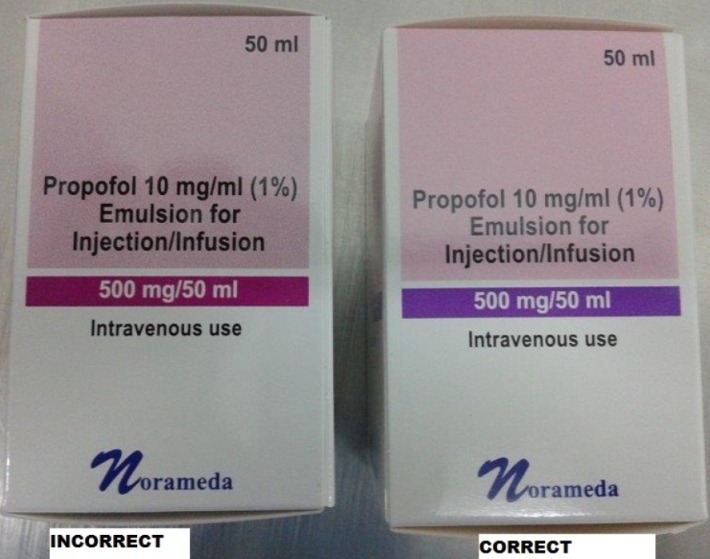
Image of Propofol 10mg/ml (1%) Emulsion for Injection/Infusion.
Download documents