Private COVID-19 testing validation
Updated 14 February 2022
Executive summary
A key part of the government’s approach to managing COVID-19 in the long term is to facilitate a thriving private sector market for COVID-19 detection tests to supplement and support testing led by NHS Test and Trace.
The government wants to encourage the private sector to bring a number of testing products and services to market to meet the differing needs of businesses and individuals. The government is keen to encourage innovation and market growth whilst ensuring tests meet minimum performance standards.
To this end the UK government proposes establishing a requirement that all COVID-19 tests placed on the UK market undergo a mandatory validation process. Tests that fail this process would be barred from sale. Retailers, distributors and manufacturers of tests that attempt to sell unvalidated tests would face sanction. This will apply equally to those companies based in the UK and overseas who are importing into the UK.
We are proposing a validation regime that would require manufacturers to register via an online portal. They would then submit data for a desk-based assessment. Once this stage had been passed, they would then provide tests to undergo independent laboratory-based validation to ascertain their specificity, sensitivity and limits of detection using range of standardised samples.
The results of all tests that pass the 2 stages of the validation process would be published providing consumers with clear comparable data against which to assess the performance of different tests on the market.
Currently the route to obtaining the data used by each manufacturer of COVID-19 tests to achieve its CE marking for COVID-19 tests is unique and designed by each manufacturer. This variability prevents consumers comparing the data and acts as a barrier to effective competition.
Manufacturers submitting tests for validation would be liable to pay a standard fee. These fees would cover the full costs of each validation.
The system would be underpinned by an enforcement regime so that unvalidated tests were identified and removed from sale, and penalties applied to retailers and manufacturers as appropriate.
If a mandatory validation requirement was to be introduced, the UK government would move quickly to make regulations under the Medicines and Medical Devices Act 2021 before the end of year in order to support manufacturers, consumers and businesses as we transition to business as usual and reopen the economy. These regulations would be focused on addressing the short-term health objective of improving COVID-19 detection tests specifically.
Introduction
As we exit lockdown in line with the government’s roadmap, we will need to remain vigilant against potential COVID-19 outbreaks.
As the market for private testing grows, it is important that consumers have confidence the COVID-19 detection tests they use give reliable and accurate results. COVID-19 remains a notifiable disease and it is important that all tests attain required performance standards.
It would undermine current public health goals if people used poor quality tests that give them a false sense of security. In the case of a false negative, this could see an infected individual unknowingly spread the virus. Conversely false positives could require business to close and people to isolate unnecessarily.
That is why government is proposing to require all tests to pass a rigorous, transparent and efficient validation, while keeping the process as agile and light touch as possible. This will ensure those tests available on the UK market from UK and overseas manufacturers are of the same quality as those purchased by the government for the NHS. As the current market drivers will act too slowly to address the immediate issue caused by COVID-19, government intervention into the market is required. This will ensure tests improve to meet our urgent health risks much more quickly. This regulatory approach will be specific for COVID-19 tests given the urgency of the intervention. It is setting a template for future regulation, however we will use learning from this regulatory regime to help us produce a world-leading best practice regulation.
This consultation covers the performance of the tests sold in the UK market both domestically produced and imported. The performance of testing service providers is assured by the UK Accreditation Service (UKAS) through a separate process that is not covered here.
We have set out below a number of questions seeking views on the government’s proposals for validation, the design of the validation process, the fees regime, and the proposed enforcement approach. The policy will be UK-wide. This is a public consultation open to everyone, including businesses involved in the manufacturer, distribution and retail of COVID-19 tests as both a good and a service from all parts of the UK. We are also keen to receive expert academic views.
Copies of the consultation paper are being sent to:
-
Association of British HealthTech Industries
-
British In Vitro Diagnostics Association
-
British Medical Association
-
Make UK
-
Medicines and Healthcare products Regulatory Agency
-
National Pharmacy Association
-
Royal Pharmacy Association
-
United Kingdom Accreditation Service
The proposals
Overarching policy objective
A key part of the government’s approach to managing COVID-19 in the long term is to facilitate and empower a thriving private sector market for COVID-19 detection tests to supplement and support NHS testing. This will support the safeguarding of public health in the long term. The government is keen to create and grow a private testing market where businesses and members of the public can trust and use the private tests they procure safely. The 2 key objectives are:
-
to ensure tests’ performance (sensitivity and specificity[footnote 1] can be relied upon by NHS Test and Trace and test users, to facilitate a robust private market
-
to grow the size of the private sector market, so a greater range of tests are available to individuals and organisations
These objectives support the government’s overarching objective of safeguarding public health.
The problem the government is aiming to solve
Consumers need to know that COVID-19 tests they buy are of satisfactory quality. This requires tests with sensitivity that is high enough to apply in ‘real world’ settings that provide accurate results and avoid spreading the virus.
Excluding self-test kits, there is currently no legal requirement available for independent validation of COVID-19 tests performance. Entry to the market is controlled by CE marking which is currently a self-declaration process for the performance of this type of test kit or equipment. This means performance is not independently verified ahead of sale.
Should COVID-19 detection tests be validated beyond the verification and assurance provided for CE marking?
- Yes
- No
If no, please provide reasoning.
As the private sector market for the manufacture and supply of COVID-19 testing grows, it is important to drive up standards of performance for tests on the market and provide clarity for consumers on the performance of tests detecting the presence of COVID-19. Removing tests from the market only after they have been reported creates a gap during which the virus could spread. This could result in local outbreaks and subsequent negative health and secondary economic impacts resulting from any necessary measures implemented to control the spread of the virus.
Driving up standards and removing poor performing tests
There is evidence that some COVID-19 tests do not perform as expected and as disclosed in their instructions for use (IFU). For example, only 25% passed through all stages of DHSC lateral flow validation to be considered of sufficient quality for procurement. Currently, (with the exception of self-certified IVDs) tests are permitted to enter the market without going through the process of an independent validation of their performance and not all tests are performing to a sufficiently high standard. This is despite 9 in 10 suppliers having the CE mark, with three-quarters meeting the ISO 13485 standard in relation to post market surveillance. Though we would expect the market to drive up quality in time, this is insufficient to meet our goals around managing COVID-19 and we need to rapidly increase quality in the private market to at least the same baseline as those procured for the NHS.
The risk of tests issuing false results could have detrimental outcomes for society and the economy. Specifically, without independent verification of private tests on the market, there is an increased risk of COVID-19 tests on the market producing the following 2 outcomes:
False negative
A person receives a negative result when they are in fact contagious and they proceed to spread the virus in the community, for example in the community, their workplaces or through social interactions. A proportion of these cases will go to develop serve disease and will require hospitalisation as well as develop long term sequalae (long COVID) with long-term health and economic impact.
False positive
A person receives a positive result when they are not infected. That person will proceed to self-isolate and the knock-on impact for individuals and businesses that have been in contact with the individual causes personal and financial costs (for example if a business is forced to close unnecessarily).
Do you agree or disagree that independently validating COVID-19 detection tests is the right approach to reduce the number of false results from tests and assure consumers that the tests they buy meet minimum quality criteria (such as specificity and sensitivity thresholds)?
- Yes, I agree
- No, I disagree
If no, please provide reasoning.
If you have evidence, you can upload a file.
Voluntary versus mandatory validation
The government has considered the comparable benefits of a voluntary validation policy compared to one that is mandatory. As the government begins to lift restrictions and non-pharmaceutical interventions (such as the lockdowns), there is a clear dependency in the government’s long-term strategy for managing the virus on ensuring that only high performing COVID-19 detection tests (which minimise the risk of false results) are available on the market. Therefore, on balance, the government has determined that it is necessary to pursue a mandatory validation scheme due to the public health risks posed by poor performing COVID-19 tests on the market.
A voluntary scheme would usually represent a lower barrier to entry into a market. However, in considering likely size of businesses involved in the market and the cost of developing a test, we do not believe a mandatory scheme would represent a significant barrier in the specific circumstances of the COVID-19 test market.
We believe that a voluntary process, discounting the cost of any fees charged, would have a significantly lower uptake than would be sufficient to provide the necessary supply of reliable high-quality tests to market. This is because the additional work and time required to submit and wait for the results of validation would be unattractive to businesses keen to get their product on the market. Particularly as manufacturers of poor quality tests would likely not apply for validation if they knew had a high chance of failing.
In a voluntary process unvalidated tests would represent a weak link that risks undermining the benefits of quality tests. As such a voluntary process would either carry considerable risk of further lockdowns or restrictions.
By comparison, a UK-wide mandatory validation scheme would be easier to deliver and would ensure all tests placed on the UK market met a minimum standard, which empowers consumers with simple and straightforward assurances of quality.
Where the requirement for validation is mandatory, we intend to make the process of transitioning as easy as possible for manufacturers to comply with and avoid circumstances where their product is not available for sale unnecessarily, for example to administrative reasons. The bar for test products to stay on the market at the end of that transition period will be for them to complete the desktop assessment stage as detailed in the Validation process section below. The intention is for the portal for submissions of the relevant data to be open for at least 4 weeks before any mandatory requirement is introduced to allow sufficient time to submit data. The announcement of the data required to be submitted will be provided ahead of the submission portal opening in order to maximise time to collate the data.
Due to the pressing urgency of managing COVID-19 pandemic and enabling people and business to return to normalcy as quickly as possible, DHSC believes a mandatory scheme is necessary.
Do you agree or disagree that a legally backed and enforceable UK-wide regime is the best approach?
- Yes, I agree
- No, I disagree
If no, please provide reasoning.
If you have evidence, you can upload a file.
Consumer information
At present there is no standardised means of comparing the performance of COVID-19 tests on the market.
The current lack of transparency over the performance of tests also has negative impacts for consumer choice. Test kit selection based on manufacturer declared performance is currently based on suboptimal, unstandardised data, making it difficult for consumers to compare performance of tests.
Currently the data used by each manufacturer to achieve its CE marking is unique and designed by each manufacturer. This allows manufacturers to tailor their use cases to ensure the most optimal performance of their tests.
This prevents consumers comparing the data and acts as a barrier to effective competition. It also means tests do not perform as well in real world scenarios as under the control conditions designed by the manufacturers.
The validation service will publish results data which will provide consumer with clear and comparable data. This will be available on the website alongside clear guidance for consumers.
Do you agree or disagree that categorising COVID-19 test performance measures as part of the validation process will ensure more standardised, comprehensive data on test performance measures from private companies?
- Yes, I agree
- No, I disagree
If no, please provide reasoning.
If you have evidence, you can upload a file.
The government’s proposal
The government is proposing establishing a regulatory requirement that all COVID-19 tests placed on the UK market must undergo a mandatory validation process that is clear, transparent, quick and efficient. More detail on the proposed process is outlined below. This will be focused solely on COVID-19 tests to help manage the immediate and urgent health need. Though as conditions change we will keep any regulatory regime we may institute under review, working with stakeholders to ensure it is still delivering to its objectives.
The scope of this proposal would include all tests – both those already on the market and those being developed and newly introduced once CE/UKCA marking has been obtained. This would apply to both domestically manufactured and imported tests. Transitional arrangements would be put in place to ensure that there was no sudden contraction in supply and those already with tests on the market would be able to continue to sell them while they are undergoing validation in the transition period.
As government is keen to maximise the supply of tests while improving quality, the regime will be as light touch and flexible as possible to minimise restrictions on tests making it to market. Imports are an important part of the UK’s supply of COVID-19 detection tests and ensuring that there is a continual healthy supply will be vital in managing COVID-19 going forward. Similarly, ensuring there are enough competitors in the market to ensure that supply, and, through competition, drive up quality and drive down prices, will be important. This is why the validation process will need to apply equally to foreign manufactures as they do to domestic ones to ensure that supply of high-quality tests. It also why the validation regime will be clear, straightforward and accessible to maximise participation from producers around the globe on an equal footing.
Tests that fail this process would be outside of the acceptable UKAS scope of tests and therefore private providers would not be approved and thus barred from sale in the UK. Retailers, distributors and manufacturers of tests that attempt to sell unvalidated test could face a number of potential sanctions based on existing enforcement powers for enforcing regulatory requirements relating to medical devices.
The results of validations would be published against performance thresholds, providing consumers with clear and comparable data against which to assess the performance of different tests on the market.
The tests currently in scope for validation cover the mature existing technology, namely antigen detection and molecular detection technologies. The most well-known examples include:
- rapid lateral flow tests (LFTs)
- polymerase chain reaction (PCR) tests
Do you agree or disagree that a mandatory validation of tests prior to their entry on to the market is best approach given the urgent need to quickly establish confidence in them as a step to reopening the economy?
- Yes, I agree
- No, I disagree
If no, please provide reasoning.
If you have evidence, you can upload a file.
Ongoing evaluation will be a key part of this regulatory regime. We will ensure any regime is adaptable and learns from best practice as it emerges in other regulatory regimes around the world. We will work with stakeholders to continually review any regulations to ensure they are still delivering a necessary intervention into the market as product quality improves and the immediate health issue subsides. Though this regulatory regime will be tied to the short-term and immediate issue of the current pandemic, we will use learning from it to help shape responses to future pandemics and other healthcare regulation particularly as people are increasingly empowered to manage their own health through accurate data.
Private testing market
The government anticipates that private sector provided testing will form a crucial part of day-to-day testing as we move into the long-term management of COVID-19 and reopen the economy. We therefore expect that this will require considerable expansion of domestic production and potentially an increase in imports to ensure there is sufficient supply to meet demand.
Naturally demand for COVID-19 tests has grown greatly since the start of the pandemic. Both in the UK and around the world frontline medical services like our world leading NHS worked tirelessly to rapidly increase testing capacity on a massive scale. This demand (though less than at the peak of the pandemic) will remain for years to come as preventative measures through testing and early identification becomes the norm in managing the virus. We expect strong demand from businesses to test employees and, in certain circumstances, customers. For these reasons, we require a strong private sector capability in testing. Though we expect demand to be for the most effective tests, and thus the market to respond to this over time with an increase in quality, this will take too long to meet our short-term public health requirement as such a market failure currently exists, and regulation can remedy in the short term.
Currently the USA is the world’s leading exporter of tests and China is quickly increasing its production particularly of lateral flow test. This creates a strong global supply in tests. However, the rapid expanse has naturally brought with it bottlenecks, for example demand for raw materials. Regulation can potentially help lead to a more productive sector. By forcing companies to focus on developing high quality tests in order to enter the market, we can expect this to mean raw material and other resources are more efficiently allocated further down the supply chain towards those companies producing higher quality tests. The government is keen to develop a resilient UK based supply chain to safeguard test supply particularly as we strive for improved quality. As imports continue, it will be important that these regulations apply equally and fairly to overseas manufacturers and wholesalers as they do to UK manufacturers and retailers.
The UK government has a number of objectives in relation to facilitating a thriving private sector market for COVID-19 detection testing. One key objective is about enabling and supporting innovation. We are keen to grow the private testing market, enabling domestic innovation to improve testing effectiveness and efficiency. The faster and more accurate tests become the easier it is to prevent the spread of infection and allow businesses, events and other organisations to operate as normal. The UK government is keen to leverage the UK’s world-renowned capabilities in medical technologies to become a world leader in the development and manufacture of COVID-19 tests. We know many companies in the UK are focusing on developing faster and more innovative testing solution as we have already seen with the development of LAMP and LamPORE assays.
As we need tests to meet a sufficient quality in order to be effective, a validation measure will only limit poor quality tests entering the market as high quality ones will already be meeting the required threshold. We do not believe that the proposed fees and timescales involved in validation would act as a deterrent to market entry for new manufacturers or cause any existing test manufacturer to leave the UK market, particularly as they would not need to compete against poor quality tests that could have lower development and production costs.
For a market to function efficiently it requires all parties to have access to information to make rational decisions. This will make the UK private testing market more competitive, as manufacturers will need to improve the accuracy and speed of their tests in order to outcompete competitors, which should continually drive innovation as well as acting as a downward pressure on prices. We do not believe validation will significantly reduce the supply of high quality COVID-19 tests on the UK market. As such, the benefits from improvement in consumer information, test quality and assurance outweigh the negligible risk of a reduction in supply.
Do you agree or disagree a mandatory validation scheme will not significantly reduce the supply of high quality COVID-19 detection tests?
- Yes, I agree
- No, I disagree
If no, please provide reasoning.
If you have evidence, you can upload a file.
Do you agree or disagree a government-run validation process will increase the likelihood of the UK being seen as a favourable place in which to carry out research relating to COVID-19 tests, develop COVID-19 tests and manufacture or supply COVID-19 tests.
- Yes, I agree
- No, I don’t agree
If no, please provide reasoning.
If you have evidence, you can upload a file.
The gold standard set by the UK government-run validation process will potentially provide recognition in export markets providing such tests with a competitive advantage against unvalidated tests. This should increase the value of tests and make the UK a key market due to the high regulatory quality, in the same way the UK’s high and effective regulation of pharmaceuticals has led to it being a global centre for drugs research and manufacture. In the US Federal Drug Administration test approval process is around £200,000 to £300,000. The UK route is likely to cost less and therefore a UK independently approved test will be appealing to global companies. We believe this should marginally increase the likelihood of the UK being seen as a favourable place to carry out research and production of COVID-19 tests.
Delivery body
The government has considered a number of potential bodies to deliver this validation service, including establishing a new agency. The assessment included the set-up costs, the length of time until the organisation could begin validating tests and the need for specialist expertise and facilities.
On this basis our analytical framework has ruled out establishing a new agency, as it would take too long to set up and develop the necessary expertise.
As we looked to build on the validation work conducted by DHSC for NHS procurement, we considered a central validation approach through DHSC expanding on that work. DHSC had the immediate skills and direct experience of the performance of COVID-19 detection tests, increasing the speed of operations being set up. This process would also allow for more rapid access to key resources, namely samples, due to the existing testing network improving economies of scale.
However, the key failure was the absence of secured laboratory space as capacity relied on for the existing work did not meet the capacity or consistency required for a mandatory program.
It was also assessed that one point of contact through the department and a single front door offered a more straightforward user experience and consistency of output to providers. This is due to the greater control that DHSC could provide over a supporting laboratory, or much smaller network of laboratories, to provide the required services.
We have also considered the Medicines and Healthcare products Regulatory Agency (MHRA) and engaged with the MHRA on developing this policy. However, centralised laboratory validation of test products is not an activity that MHRA conducts. Where similar activities are undertaken for third party conformity assessments for CE marking, these are conducted by approved independent bodies. The ability to scope a validation process, prepare this and then initiate activities would have required significant immediate investment in resourcing for MHRA.
Finally, as the designated authority that administers and enforces the law on medical devices in the UK, MHRA would potentially face a conflict of interest in defending its own validation assessment, should a complaint arise about a device in scope of the regulation. Avoiding the risk of potentially undermining the independence of the UK regulator was a key reason for MHRA not owning this process.
A further alternative considered was to build a framework standard for laboratories to comply with in order to conduct the validation work on behalf of the manufacturers and provide an independent evidence base for the test product’s performance. Whilst this offers a lower risk to government in terms of not owning the outputs of the validation process and relying on existing laboratory capacity, there are significant downsides to a decentralised approach.
It was assessed that the process was unlikely to be able to be set up within the required timescales and that maintaining oversight of the quality of the outputs from laboratories would require significant monitoring resource and cost, as well as the collation of the data for publication. Furthermore, any support in relation to set up costs to enable the labs to have the right equipment would necessarily be duplicated and economies of scale in other areas would be missed.
Based on our analysis, we believe a central approach through DHSC and a supporting laboratory would be the best option to deliver this validation service, as this option would fit most easily with the department’s usual operations and builds on the expertise and knowledge already held in the department.
Do you agree or disagree that the Department of Health and Social Care is the appropriate delivery body to provide UK-wide validation?
- Yes, I agree
- No, I disagree
If no, please provide reasoning.
If you have evidence, you can upload a file.
Validation process
The government wants to create a process for validation which is robust and fit for purpose, and efficient and accessible for end users. It will treat products and producers the same regardless of origin as we are keen to encourage producers from all over the world to validate their tests and supply the UK market. An overview of the expected process for validation is set out below.
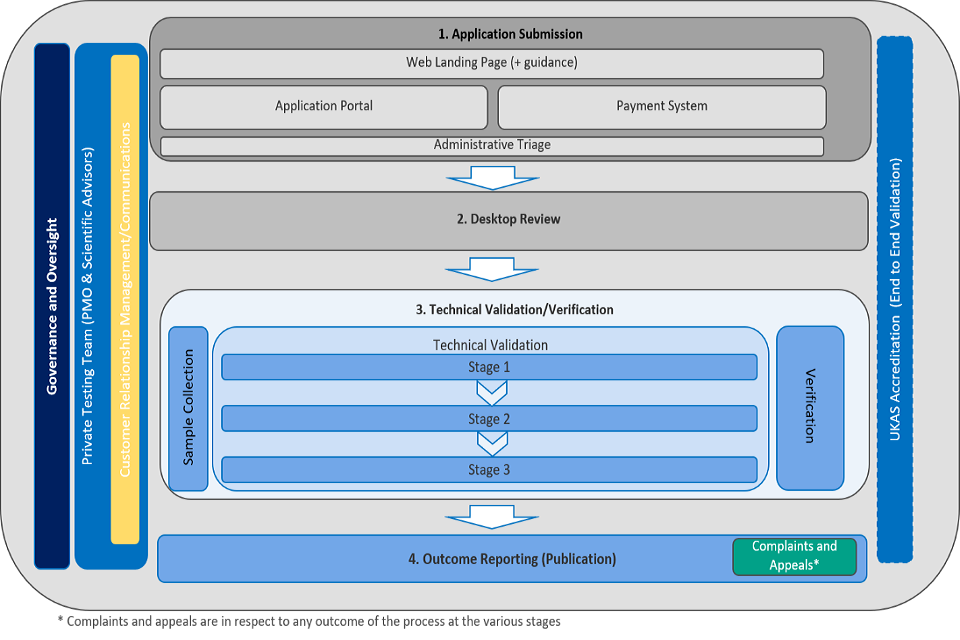
Under a mandatory validation regime, a manufacturer of a testing product or testing service who intends to supply that product or service for human use would be legally required to have validated that test by DHSC before it could be placed on the market.
This will require the company or individual to submit an application for validation of their test via a web portal. This will start a multiple step validation process. As part of their application, they will be required to pay a fee through a link to a GOV.UK Pay portal on the same GOV.UK website. Payments will be made to match the stages of the validation process. In addition to the application and payments, the site will contain relevant guidance on our processes and services.
Once the application has been received by the validation service it will be placed into administrative triage.
Tests will then enter either a 1-step or a 2-step process.
1. Desktop review
An assessment of the self-declared information from the applicant will be undertaken by a scientific advisor. This will take the form of a half-day desktop review to ensure that the application is complete and meets the minimum standards through a structured gap analysis. This will be supplemented by a check as to whether this test has passed or failed DHSC’s validation process for lateral flow devices or other COVID-19 detection tests for NHS procurement purposes. On successful completion of the review, a bespoke technical validation protocol will be developed to address the identified gaps. The outcome of the desktop review is taken to an expert panel for quality assurance and following this to DHSC’s Technical Validation Group (TVG) for further endorsement prior to going to the Technologies Validation and Assurance Board for final approval. On approval the laboratory undertaking the technical validation will be informed and the bespoke validation protocol shared.
2. Technical verification
This will involve lab-based testing of the test, to establish its specificity and sensitivity thresholds when used as the manufacturer has indicated it should be used. This will happen over 3 stages. Products completing all 3 stages will have completed validation against the required set of samples in line with the MHRA recommended levels for such a process. This would be the full extent of the proposed assurance and is in line with the MHRA recommended levels. This validation process will ensure that we have a degree of assurance over the performance of a test to be reasonably confident it can be sold.
The details of that test’s validation results will be sent to the TVG and expert panel and then provided to the applicant. Following this the applicant will have the opportunity to lodge an administrative appeal about the outcome. At any time during the process the applicant will be able to make a complaint about service, timescales, and so on. Once this process has been completed the results will be analysed and outcome will be published. This will provide information to consumers to inform their decisions and allow them to check the validation status of tests they may purchase.
Guidance for employers and end users – we will publish this will be broken into 4 categories of use with set minimum thresholds for each use scenario. Lists of tests will also be published for reference by people choosing which test to use.
Do you agree or disagree that the proposed mandatory validation process set out in the consultation document will increase the safety of COVID-19 tests and reduce the risks presented by poor quality tests?
- Yes, I agree
- No, I disagree
If no, please provide reasoning.
If you have evidence, you can upload a file.
Do you agree or disagree that a desktop review followed by a laboratory testing is the most effective way to validate tests?
- Yes, I agree
- No, I disagree
If no, please provide reasoning.
If you have evidence, you can upload a file.
How much time do you think is reasonable to complete an application to validation? Please explain.
- (Free text entry)
Do you have any further comments you would like to make about your expectations around the validation process or user experience?
- (Free text entry)
Fees
The government’s intention is this validation system will be cost neutral with all costs recovered through fees paid by applicants. This will mean costs fall fairly on manufacturers rather than taxpayers.
The UK government estimates suggest that a fee of between £55,000 and £75,000 for full validation of a product at both the desktop and technical assessment (laboratory) levels will ensure that costs are recovered.
Fees will be payable in 2 stages:
- an initial payment will be taken for desktop review of the documentation submitted by the manufacturer covering the costs of the portal and time required to review. As such this will be a smaller share of the full fee
- the remaining costs will be charged for entering the second phase of the validation process (technical assessment in the laboratory) which due to the requirement for specialist equipment and staff as well as samples of the virus represent the majority of the total fee
This is designed so that manufacturers will not pay for a full laboratory validation if their product does not proceed past initial desktop review minimising the financial burden on applicants whilst also fully funding the services undertaken on their behalf.
As the majority of costs at each stage are resource costs, in the event that a product fails at any stage and the manufacturer reapplies after improvements or changes. This would be treated as a new application and the full cost for each stage would apply again.
We do not want fees to present a barrier to entry for either UK or overseas-based manufacturers. We particularly do not want them to prevent SMEs bringing innovative new testing solutions to market. As such, we could consider a range of policy options to mitigate the cost impact to smaller market participants while still being fair to the taxpayer. So we would particularly welcome evidence on the potential impacts on SMEs in respondents’ evidence.
Do you agree or disagree that the costs of the validation process and associated running costs should be covered through fees rather than subsidised by the taxpayer?
- Yes, I agree
- No, I disagree
If no, please provide reasoning.
If you have evidence, you can upload a file.
Do you think that the proposed fee between £55,000 and £75,000 per test for the total cost of the validation service is reasonable?
- Yes
- No
If you answer no, please explain how much you would be willing to pay for this service and provide your reasoning.
If you have evidence, you can upload a file.
Can you name other national or international diagnostic testing validation services that require payment, and what the cost is of those services?
- (Free text entry)
Enforcement
For a mandatory regime to be effective there must be enforcement framework with an ‘enforcement authority’ able to exercise proportionate powers to investigate and address suspected non-compliance, remove unvalidated tests from the market and sanction offences in a proportionate manner. We consider that a person or organisation that commits an offence under the regulations should be liable to civil or criminal sanctions as set out below.
If the validation regime was made mandatory by making regulations under the Medicines and Medical Devices Act 2021, the validation requirements would be enforced using the same enforcement powers in place for enforcing the Medical Devices Regulations 2002. This section explains those in more detail.
Enforcement authority
In line with existing enforcement practice for medical devices the enforcement authority will be the Secretary of State for all medical devices and in relation to devices that are consumer products it will be a local weights and measures authority. In practice this will mean that the MHRA, and local authority Trading Standards would be responsible for enforcement as this will build on their existing functions and capabilities and should avoid unnecessary costs and duplication.
Enforcement powers
It will be important for the enforcement authority to be able to undertake investigations to obtain information about suspected offences to enable it to exercise enforcement powers in an appropriate and proportionate manner. To facilitate this, the enforcement authority would have the power to issue information notices to any person that it believes may have information that it requires in order to determine what further action to take.
The information notice will require a person to produce records or information specified information to the enforcement agency within a set timescale and enable a person appointed by the enforcement authority to take copies of that information. This information will be used to support an evidence led enforcement process.
The enforcement authority will in the first instance use a range of notices to force retailers or manufacturers to take specific action;
-
compliance notices – this will enable the enforcement agency to require the manufacturers, retailers or persons involved in supply to take measures to comply with a validation provision by a specified deadline and to provide evidence they have done so
-
suspension notices – this will allow the enforcement agency to prohibit a business from range of actions related to supplying a COVID-19 test or agreeing to supply tests in the future for a temporary period of up to 6 months
-
safety notices – this would build on the powers in the suspension notice to also include the power to force a business to publish warnings, contact customers and run a product recall
These notices will set out the grounds for the action being taken and provide an opportunity for the affected business or person to appeal the notice and its terms.
We believe this range strikes the right balance between giving enforcement authority the right toolbox to deal with a broad range of scenarios in a flexible and agile way, while working positively with industry and keeping enforcement light touch as far as possible.
Civil and criminal liabilities
To ensure compliance with the proposed validation requirements we propose that breaching a requirement should amount to a criminal offence and that the enforcement authority should be able to apply a range of different sanctions so that, where enforcement action is necessary, it is proportionate to the offence in question. Therefore, we suggest that the enforcement authority should be able to use following range of sanctions:
-
forfeiture of goods – allowing the seizure of unvalidated tests
-
monetary penalties – these are civil sanctions which would be classed as debt to the Secretary of State
-
fines – fines levied on individuals or companies following prosecution
-
custodial sentences – for individuals or corporate officers on summary conviction in England and Wales, to imprisonment for a term not exceeding 51 weeks (this is limited to 6 months until section 281 of the Criminal Justice Act 2003 is in force), in Scotland or Northern Ireland, to imprisonment for a term not exceeding 6 months
Given the importance of COVID-19 tests being reliable and the severe impacts poor tests can have on the national recovery from the COVID-19 pandemic, particularly with the potential to cause local outbreaks with the resultant loss of life, we consider it is appropriate that a breach of a validation requirement, for example placing a test on the market before it has been through the validation process or selling an unvalidated test, should constitute a criminal offence.
Do you agree or disagree that the range of enforcement powers set out above is appropriate and proportionate as a first line response for breaches of regulation that may arise?
- Yes, I agree
- No, I disagree
If no, please provide reasoning.
If you have evidence, you can upload a file.
Do you suggest any other additional powers are required to investigate suspected offences?
Do you agree or disagree that MHRA and local authority Trading Standards are the correct enforcement authorities?
- Yes, I agree
- No, I disagree
If no, please provide reasoning.
If you have evidence, you can upload a file.
Do you agree or disagree that the failure to comply with the enforcement notices should be a criminal offence?
- Yes, I agree
- No, I disagree
If no, please provide reasoning.
If you have evidence, you can upload a file.
Next steps
Following the closure of the consultation period on the 5 May DHSC will analysis the responses to inform policy making. If the decision is taken to introduce a mandatory validation process, we expect to move quickly towards laying secondary legislation. The UK government response to this consultation will follow in due course.
As a COVID-19 test is a medical device used for diagnosis, the UK government proposes using the Medicines and Medical Devices Act to establish a regulatory regime which will require manufacturers of COVID-19 tests to independently validate the performance of their tests in order for these to be placed on the UK market.
This will place a legal duty for manufacturers to go through the validation process before supplying COVID-19 tests. It would be a criminal offence for manufacturers, vendors or distributors to sell unvalidated tests.
The UK government is also considering introducing a ‘manner of sale’ statutory instrument (SI) which will require a notification before a purchase is made from a manufacturer or distributor. This will be an effective tool in raising awareness among people purchasing COVID-19 detection tests.
Prior to legislation coming into force, we would progress with a voluntary validation program.
We intend to keep the regulatory regime under review and engage continually with stakeholders to reform it as necessary in response to feedback. We believe this flexible approach will keep the regulation sufficiently agile to respond to evolution in the market whilst ensuring high standards are maintained continuously.
Do you feel the proposed regulations are fair for businesses?
- Yes
- No
If no, please provide reasoning.
If you have evidence, you can upload a file.
Do you have any additional comments to add to the response?
If you have any questions, or you would like to submit evidence via email, please contact [email protected].
Annex A: summary of the Secretary of State’s assessment of the matters set out in section 15 of the Medicines and Medical Devices Act 2021
The Secretary of State’s overarching objective in making any regulations under section 15 of the Medicines and Medical Devices Act 2021 to give effect to the policy outlined in this consultation document would be to safeguard public health.
In considering this policy and the regulations which may be needed to give effect to it, the Secretary of State has had regard to:
The safety of medical devices within the scope of this policy
The safety of the testing kits themselves is unlikely to be effective as they do not represent a health risk in and of themselves to an individual if misused. They do however present a risk to the general public health as poor information can lead to the virus spreading.
Any impact of this proposal on the safety of the COVID-19 tests will be positive, since it will ensure that only tests meeting a minimum standard of accuracy are available on the UK market. It is therefore considered that this proposal does not create any risks for the safety of medical devices. The rigorous validation process set out above ensure test of are of sufficient quality to protect the public health.
The availability of medical devices within the scope of this policy
Creating a new regulatory standard will limit those tests that wouldn’t meet this standard from entering the market. This in theory will reduce the number of test available to the UK market. However, in reality we are removing tests from circulation that could be a threat to public health due to their higher than tolerable propensity to give false results. Given the impact false negatives can have on the public health as users can unwittingly infect others having been given the confidence not to self-isolate by a poorly performing test justifies removing them as they can in reality be worse than useless and present a harm to the public health.
I do not believe the type of validation we are proposing nor the fees or time the process will require, will act as a deterrent to manufacturers of quality tests, given both the size of the businesses involved and their experience with dealing with such processes.
The creation of a highly respected validation process could create a market efficiency with high quality tests seeking the UK market and poor quality tests self allocating themselves out, or striving to raise their standards to a sufficient level. In either scenario we the supply of high quality tests is unlikely to be impacted.
Whether the United Kingdom is likely to be seen as a favourable place in which to: research the medical devices within the scope of this policy, develop medical devices within the scope of this policy or manufacture or supply medical devices that come within the scope of this policy
As set out in the qualitative analysis in the private market section, I do not believe a validation regulatory regime of this kind will deter any business from researching or manufacturing COVID-19 tests in the UK.
It may dissuade suppliers of low quality tests from bringing stock to the UK, however I do not consider this to be a negative impact but a positive as we wish to focus our market solely on high quality tests.
Good regulation will give business clear and consist rules this is something all businesses rely on. I fully intend for the resulting SI to be just such an example of good regulation.
The Secretary of State having assessed the above criteria and his overarching objective to safeguard the public health. Notes the minor risk to the supply of COVID-19 tests but believes this is negligible in regard to tests of sufficient quality to be an effective tool in identifying infections early. The removal of poor quality tests is a more substantial benefit to the protecting public health.
I intend to have a full impact assessment developed going forward which will be able to provide further analysis regarding question 2 and question 3.
The Secretary of State will continue to assess the matters set out in section 15(2) to (4) before making any regulations under section 15 to give effect to the policy proposed in this consultation.
-
Sensitivity measures how well a test can identify true positive samples. Poor sensitivity gives rise to false negative results. Specificity measures how well a test can identify true negative samples. Poor specificity gives rise to false positive results. Limit of detection is the minimum concentration of virus or viral material that can be reliably detected. The lower the limit of detection, the greater the sensitivity of a test. ↩
