Carprieve Tablets 50mg - Product defect recall alert
Details of product recall for Carprieve Tablets 50mg Vm 02000/4221 by Norbrook Laboratories Limited.
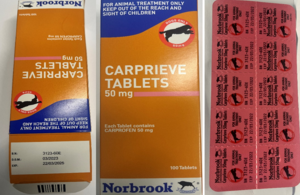
We wish to make wholesalers and vets aware that Norbrook has initiated a Class III recall to wholesaler level.
The reason for the recall is due to the incorrect expiry date of 22/03/2022 printed on the blisters of Carprieve 50mg Tablets, Vm 02000/4221, Batch Number 3123-60E.
The correct expiry of the blisters is printed on the outer carton which is 22/03/2025. This issue is isolated to the above-mentioned batch only.
A caution in use letter will be sent to veterinary practices who are in receipt of this batch explaining that the expiry date as printed on the carton is the correct date and should be followed.
For any customer queries please contact, QA Department by telephone on +44 (0)28 3026 4435 or email [email protected]