Prednicare 1 mg Tablets - Product recall alert
Product defect recall alert for Prednicare 1 mg Tablets Vm 32742/4034 by Ecuphar N.V.
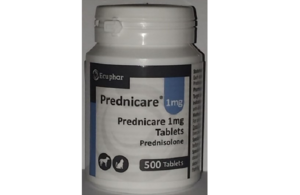
Container of Prednicare 1 mg Tablets
We wish to make wholesalers/distributors, veterinarians and end-users aware that Ecuphar N.V. has initiated a Class II recall for Prednicare 1 mg, 500 tablets.
This recall is due to the presence of 5 mg Prednicare tablets found in 2 pots of Prednicare 1 mg. This recall applies to the following batch only:
| Product | Batch No. | Expiry date |
|---|---|---|
| Prednicare 1mg, 500 tablets | 24B01 | 02/2027 |
This contamination involves a clear risk of overdose of 5 times the recommended dose. However, up to today no adverse events linked to the affected batch number and defect were reported.
Overdose signs are detailed in the product information leaflet, under ‘Special Warnings’; ‘Overdose’.
Ecuphar N.V. is contacting distributors/wholesalers and veterinarians to examine inventory immediately and quarantine products subject to this recall.
End-users should return prescribed tablets of Prednicare 1mg, batch 24B01 only, to their veterinary surgery.
For further information regarding the recall, please contact Animalcare Ltd via email at [email protected] or telephone 033 0818 9717.