Short protocol for the enhanced surveillance of childhood cases of hepatitis B and C in England
Updated 5 August 2022
Applies to England
For the Hepatitis Infection Paediatric Surveillance Network (HIPSNet), a collaboration between: clinical paediatric infectious disease and hepatology specialists and the Immunisation and Vaccine Preventable Diseases Division and Virus Reference Department of the UK Health Security Agency (UKHSA).
With the introduction of a hexavalent vaccine containing hepatitis B into the primary immunisations as part of the routine (universal) infant programme in England in mid-2017 and the availability of direct acting antivirals for treatment of hepatitis C in children, requirements for the surveillance of hepatitis B and C cases have changed. In order to monitor the performance of the vaccination programme more detailed information is required on childhood cases of hepatitis B diagnosed following the programmes’ introduction. There is also a need to monitor treatment and outcome of chronic cases of hepatitis B and C to ensure equitable access to treatment and care.
1. Aims and objectives
The aim of the enhanced surveillance of childhood cases of hepatitis B and C is to collect more detailed information on confirmed cases of hepatitis B in children aged under 17 years old in order to monitor the effectiveness and impact of the routine childhood vaccine programme, monitor risks and trends in children diagnosed with hepatitis B and C, monitor the uptake and outcome of testing and treatment interventions and care quality and equity.
The objectives of the enhanced surveillance of childhood cases of hepatitis are to:
- describe the epidemiology including demographic, risks, sources of infection and clinical features of childhood cases
- determine the HBV genotype and undertake molecular characterisation of viruses found in childhood cases,
- determine vaccination status to identify if cases represent vaccine failures or missed opportunity for testing and vaccination
- identify potential barriers to testing, vaccination and treatment.
- evaluate infant testing and vaccination and new entrant screening and vaccination
- ensure children with hepatitis B and C are referred to specialist care and monitor equitable access to services
- monitor and review outcomes of care and treatment to identify best clinical practice to share information between units and regions
- monitor demand on paediatric infectious disease services and facilitate healthcare planning to reduce future burden
- reduce inequalities in care due to the diverse needs of different populations and the wide range of specialists involved in treatment pathways
- feedback findings to clinicians to improve standards for testing, vaccination and treatment
2. Methods
2.1. Case reporting to UKHSA
There are 4 routine sources of case reports of hepatitis B and C to the Hepatitis team at UKHSA Colindale: data from the UKHSA laboratory reporting surveillance system Second Generation Surveillance System (SGSS), data from HPZone, the case management system used by Health Protection Teams, the enhanced surveillance of infants born to hepatitis B positive mothers, which is fed into by the Integrated Screening Outcomes Surveillance System (ISOSS) and notifications directly from clinicians managing paediatric hepatitis B and C infections.
-
The SGSS is a national surveillance system that obtains records of all positive microbiological and virological test results from NHS diagnostic laboratories, and some private laboratories, contains all laboratory confirmed cases of diagnosed hepatitis B and C.
-
HPZone is the case management system used by the Health Protection Teams in UKHSA. This is mostly a source of acute hepatitis B cases as these are prioritised for public health action.
-
The Enhanced Surveillance of Infants Born to Hepatitis B positive mothers is a system monitoring the hepatitis B antenatal screening and selective neonatal immunisation pathway in partnership with NHSE and ISOSS, any mother-to-child transmission events identified in this pathway will be reported.
-
Online survey forms are available and distributed to clinicians known to be directly managing paediatric hepatitis B and C infections so cases can be reported directly.
2.2. Co-ordination of surveillance activities
Coordination of the enhanced surveillance, including analysis and outputs will be undertaken by the Hepatitis team of the Immunisation Vaccine Preventable Diseases division and the Virus Reference Department at UKHSA Colindale.
2.3. Case definition
To be included in this surveillance system a case must meet the following eligibility criteria:
- laboratory confirmed hepatitis B or C infection (acute or chronic)
- aged under 17 years old upon first notification
- resident in England
- reported to UKHSA Colindale through the SGSS laboratory surveillance system, HPZone, ISOSS, or directly from a treating clinician
2.4. Data collection
2.4.1. Case identification
Cases are extracted from all UKHSA sources on a monthly basis and fed into a central database. Case notifications are received from:
- Second Generation Surveillance System (SGSS) – UKHSA’s national laboratory surveillance database
- Integrated Screening Outcomes Surveillance Service (ISOSS) – mother-to-child transmission events.
- HPzone – UKHSA’s Public health case and outbreak management system.
- snap survey – case reports directly from specialist paediatric clinicians.
- the National Reference Laboratory
Cases from the national laboratory surveillance database are extracted on the following criteria:
For hepatitis B
The extract is restricted to confirmed cases only based on the following case definitions:
Acute - HBsAg positive and anti-HBc IgM positive.
Chronic - HBsAg positive twice at least 6 months apart or HBsAg positive and anti-HBc IgM negative and anti-HBc positive.
Based on the laboratory markers therefore a provisional ‘acute’ or ‘chronic’ diagnosis should be available for all confirmed cases, however incomplete data can mean that this diagnosis is often uncertain. Uncertain diagnoses will be followed up for confirmation with relevant specialists.
For hepatitis C
The extract is restricted to hepatitis C PCR positive cases only.
2.4.2. Clinical and epidemiological data collection
Upon notification, confirmed childhood hepatitis cases will be run through the Personal Demographics Service (PDS) to obtain the patient’s full contact details and the details of their general practitioner (GP). The patient will also be cross-checked against other linked data sets to import information already known about the case.
For cases not directly reported by specialist clinicians, letters will be sent to the patient’s GP first asking them to confirm the infection status if possible and to notify UKHSA of the specialist managing the child’s infection. In addition, for cases of hepatitis B GPs will be asked to complete a baseline Enhanced Surveillance of Childhood hepatitis B cases questionnaire. This questionnaire includes questions on the case’s hepatitis B vaccination history, clinical symptoms, laboratory results; demographic information, information on potential source of infection.
A follow-up telephone call will be made to non-responding GP practices.
For both hepatitis B and C cases, the named clinician managing the hepatitis infection will be contacted informing them of the childhood surveillance programme and asking for completion of a baseline clinical data form. If it is identified that a child’s infection is not being monitored or managed, UKHSA will provide patient information to the HIPSNet regional clinical lead who will liaise with the GP to try to ensure access to care (see Appendix 3b). Neither UKHSA or the corresponding member of HIPSNet assume clinical responsibility for the children identified through this surveillance.
Annual follow up questionnaires will be sent to the named lead consultant responsible for the care of child’s hepatitis, usually Paediatric Infectious Diseases or Gastroenterology Hepatology Specialist. These will be sent out until a child moves into adult care, their infection resolves or they are lost to follow-up (that is, moves out of England). Follow-up questionnaire data will be entered via a web portal (snap survey).
Where information on household contact and child vaccination status is not available from the GP, these will be requested from the specialist managing the child’s infection through a supplementary form at first follow-up.
2.4.3. Molecular characterisation of new hepatitis B cases
Where hepatitis B cases are notified through laboratory surveillance, residual blood samples will be requested to be sent from the laboratory of diagnosis to the Virus Reference Department, UKHSA Colindale (if not already requested via the existing enhanced surveillance for acute hepatitis B).
Residual samples will only be requested for those under 10, as the primary aim of their collection is in evaluating our UKHSA’s infant vaccination programmes. Samples received at the reference laboratory will undergo molecular characterisation investigations to confirm the diagnosis and conduct additional genotyping and phylogenetic analysis. Genotyping and phylogenetic analysis provide information on the diversity of the viruses affecting children with additional molecular characterisation informing on the presence of variants.
2.6. Information governance
UKHSA has legal permission, provided by Regulation 3 of The Health Service (Control of Patient Information) Regulations 2002 to process patient confidential information for national surveillance of communicable diseases. This includes UKHSA’s responsibility to monitor the safety and effectiveness of vaccines, identify risks and trends in diseases and outcomes, and as such, individual patient consent is not required. The UKHSA Colindale Information Governance leads have approved the inclusion of this surveillance system under the above-mentioned legal framework.
Patient identifiable information may be shared with members of HIPSNet clinicians only if it is identified the child is not currently in direct care for their hepatitis infection. Aggregated national surveillance data will be shared with the Paediatric Infectious Diseases or Hepatology Consultant responsible for managing the child’s hepatitis infection.
A privacy notice can be found in (see Appendix 4) which gives further details on information governance and some frequently asked questions on data collection.
2.7. System level security policy
A system level security policy (SLSP) has been drafted for this additional enhanced surveillance system and has been reviewed and approved by UKHSA information governance leads.
3. Dissemination of information and outputs
If this surveillance programme identifies any cases where a health protection incident is considered to have been the most likely source of infection, for example during healthcare procedures in the UK, this will be reported promptly to the local health protection team (HPT). The HPT will be responsible for any incident investigation and follow-up.
Annual reports on aggregated national data from the treatment and outcome questionnaires will be disseminated to reporting paediatric infectious diseases and hepatology consultants.
4. Appendices
4.1 Appendix 1. Enhanced surveillance questionnaires
1a) Questionnaire for GPs
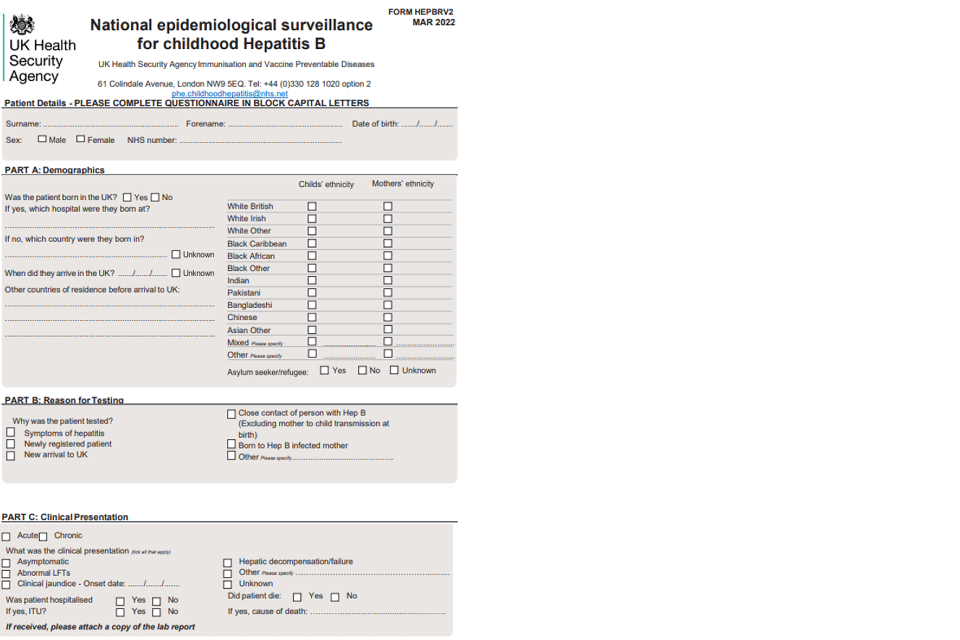
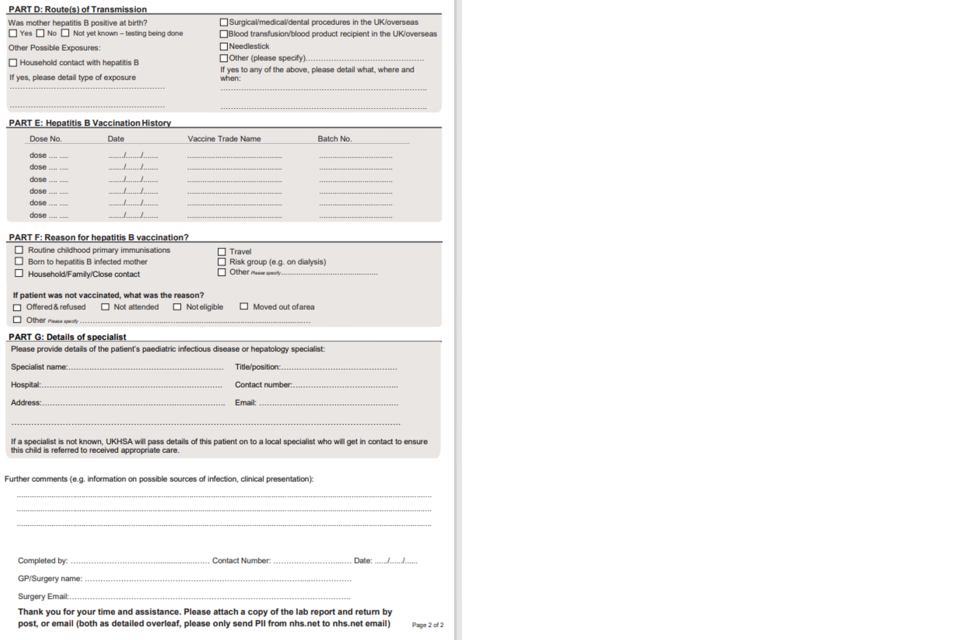
1b) Links to enhanced surveillance questionnaires for paediatric specialists
4.2 Appendix 2. Letters and communications
2a. Letter general practitioner to request baseline questionnaire
Re: Enhanced surveillance of childhood cases of hepatitis B in England
The above-named child has been diagnosed with hepatitis B according to NHS laboratory data notified to the UKHSA. The UKHSA is following up all children under 17 years old who have been diagnosed with hepatitis B as part of the UKHSA’s duty to monitor the effectiveness of hepatitis B combination vaccine (DTaP/IPV/Hib/HepB) introduced into the routine childhood programme in autumn 20171, as well as care quality and outcomes.
Therefore we kindly request that you:
- Complete the attached surveillance questionnaire form.
- Attach a copy of the patient’s laboratory report with full hepatitis B markers.
The information you provide will be used to monitor the impact of the vaccination programme and inform changes to the routine childhood and selective neonatal hepatitis B programmes. If there is any doubt about the diagnosis of hepatitis B in this child, please let us know so we can verify data and correct our records.
May we also remind you that all children with new diagnosis of hepatitis B should be referred to a liver specialist for assessment and further management.
Close household and family contacts should be tested and vaccinated as per national recommendations in the Green Book.
The information you provide is for surveillance purposes only and will be treated in strict medical confidence. This data is collected by the UKHSA under Section 251 of the NHS Act 2006 and the Health Service (Control of Patient Information) Regulations 2002 (‘section 251 support’) which provides cover to process confidential patient information for the purposes of monitoring the efficacy and safety of the vaccination programme with exemption for the requirement for consent. UKHSA’s Personal Information Charter explains how we protect personal information.
We are very grateful for your continued cooperation in delivering immunisation programmes and supporting national surveillance for vaccine preventable infectious diseases. If you have any queries please contact us on the telephone number or email above.
Footnote 1. All babies born on or after 1 August 2017 are eligible for a hexavalent vaccine (DTaP/IPV/Hib/HepB) which includes hepatitis B for their primary immunisations. The routine infant immunisation schedule remains unchanged at 8, 12 and 16 weeks of age. All babies born to hepatitis B infected mothers on or after 1 August still need to receive a monovalent hepatitis B vaccine dose at birth and 4 weeks of age. They should then receive hexavalent vaccine at 8, 12, and 16 weeks and have a final dose of monovalent hepatitis B vaccine at one year of age, at the same time as a HBsAg test to exclude chronic infection.
2b. Letter to laboratory of diagnosis to request residual blood samples
Re: Enhanced surveillance of childhood cases of hepatitis B in England
Dear colleague
The enhanced surveillance of childhood cases of hepatitis B has been implemented to monitor the impact of the introduction of a hexavalent vaccine containing hepatitis B into the routine childhood immunisation programme in autumn 2017.
As part of this surveillance, residual blood samples from newly diagnosed cases of childhood hepatitis B are requested from the laboratory of diagnosis to permit molecular characterisation investigations at the Blood Borne Virus Unit (BBVU), Virus Reference Department (VRD), UKHSA Colindale. BBVU analysis of samples from cases will allow population based monitoring of virological factors associated with infection, including breakthrough infections in vaccinated children. This information will be combined with the demographic and risk factor information from GP reporting to monitor and inform changes to the immunisation programme.
All samples submitted will undergo HBV genotype assignment with additional sequence analysis to determine the presence of amino acid changes across the HBsAg region.
(Amino acid changes resulting in the abrogation of the HBsAg conformation leading to an alteration in HBsAg antigenicity have been associated with variant viruses and vaccine escape).
Laboratories are requested to use the enclosed request form to send in the samples to UKHSA Colindale to ensure that they are not charged. Epidemiological information will be obtained from parallel reporting from GPs so sufficient identifiers are required for accurate matching.
Information on the genotype and mutational analysis will be returned to you within 3 weeks. The sequence information will be added to the national HepSeq database. This resource is supported by UKHSA for surveillance and research into hepatitis B.
2c. Letter to hepatitis specialist informing of diagnosis
Re: Enhanced surveillance of childhood cases of hepatitis in England
The above-named child has been diagnosed with hepatitis according to NHS laboratory data notified to UKHSA. UKHSA is following up all children under 17 years old who have been diagnosed with hepatitis (B or C) UKHSA are also monitoring access to care, outcomes and treatments in childhood cases of hepatitis B and C to reduce inequalities in care across this population.
UKHSA has sent a questionnaire to the patient’s GP where you were named as the patient’s specialist and would be best suited to provide information on this child’s care. We would be grateful if you could confirm you are willing to do this for the patient. If you are, we will send a link to your email address (specialist address) to complete a baseline questionnaire and then once annually asking to complete a short online follow questionnaire on this patient.
Please let us know if this child does not have a hepatitis (B or C) diagnosis or clears their infection so we can cease follow-up of this case.
If you need to contact us regarding this case, please email: [email protected] or, if you are sending sensitive or confidential information: [email protected]
2d. Letter informing child not in specialist care
Re: Hepatitis B infected child not in specialist care
Dear colleague
The above-named child has been diagnosed with hepatitis (B or C). UKHSA is following up all children diagnosed with hepatitis B or C infection as part of the UKHSA’s duty to monitor and review access to care, treatment and outcomes, as well as to reduce inequalities in care.
UKHSA has been informed this child is not currently under specialist management for their hepatitis infection. You have been identified as the geographically the most suitable member of the Hepatitis Infection Paediatric Surveillance Network (HIPSNet) to be informed of this case. This does not constitute a transfer of care and neither UKHSA nor yourself are taking responsibility for this child’s care at this time.
We recommend as per HIPSNet surveillance protocol, that you contact the patients GP detailed below to try to devise a strategy to get the child into specialist care. Should you be successful in getting this child into care, please can you can notify UKHSA at [email protected] providing details so further surveillance can begin.
4.3. Appendix 3. Protocol flow-charts
3a. Flowchart of communications and questionnaires sent by notification source for hepatitis C
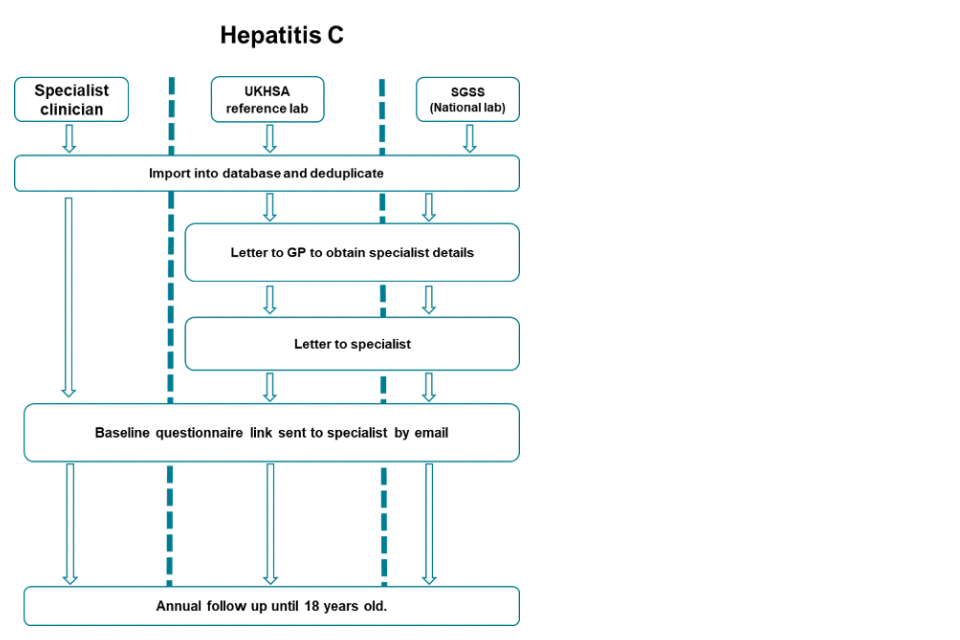
Accessible text equivalent of flowchart 3a
There are 3 pathways: specialist clinician, UKHSA reference lab and SGSS (national laboratory). For each pathway firstly cases are imported into a central database and deduplicated.
For any new cases from UKHSA reference lab or SGSS pathways, letters are sent to the GP requesting details of the specialist. Letters will then be sent to this specialist followed by a baseline questionnaire link sent by email.
Cases notified by the specialist clinicians will be sent the baseline questionnaire straight away.
For all pathways, annual follow-up will occur until the child is 18 years old.
3b. Flowchart of communications and questionnaires sent by notification source for hepatitis B
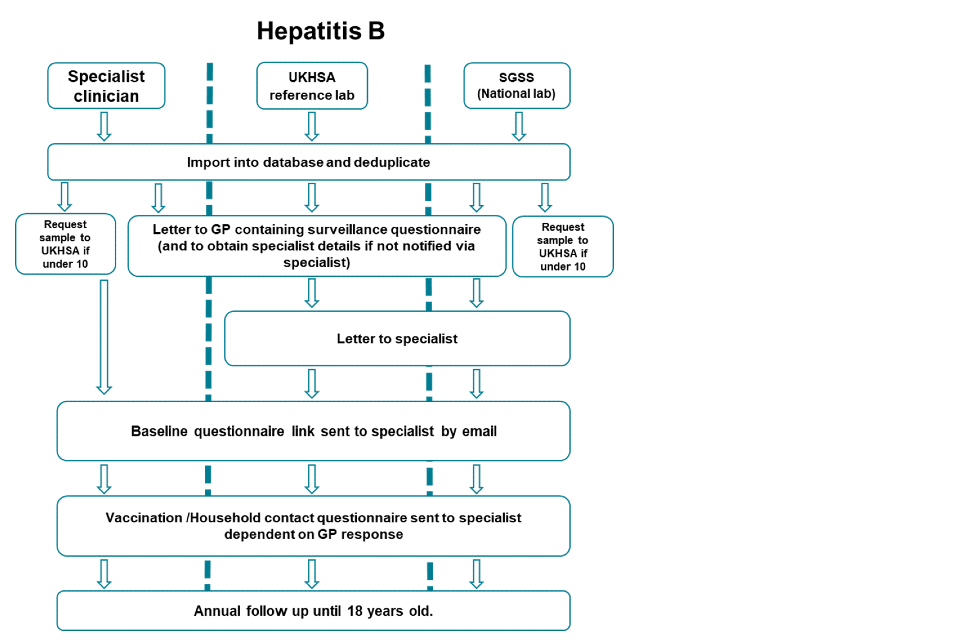
Accessible text equivalent of flowchart 3b
There are 3 pathways: specialist clinician, UKHSA reference lab and SGSS (national laboratory).
For all pathways, firstly cases are imported into a central database and deduplicated.
If child is under 10 and the notification is from a specialist clinician or SGSS, a letter will then be send to the local lab requesting they refer a sample to UKHSA.
In all 3 pathways, a letter will be sent to the GP asking for completion of a surveillance questionnaire and to notify UKHSA of the specialist details (if not notified directly by the specialist already)
For notifications only from UKHSA reference lab and SGSS, a letter will then be sent to the specialist.
Following this, in all 3 pathways, a baseline questionnaire link will be sent to the specialist by email, followed by a vaccination and household contact questionnaire dependant on the response from the GP.
Annual follow-up will then occur in all 3 pathways until the child is 18.
3c. Protocol for hepatitis B or C cases not currently under specialist care
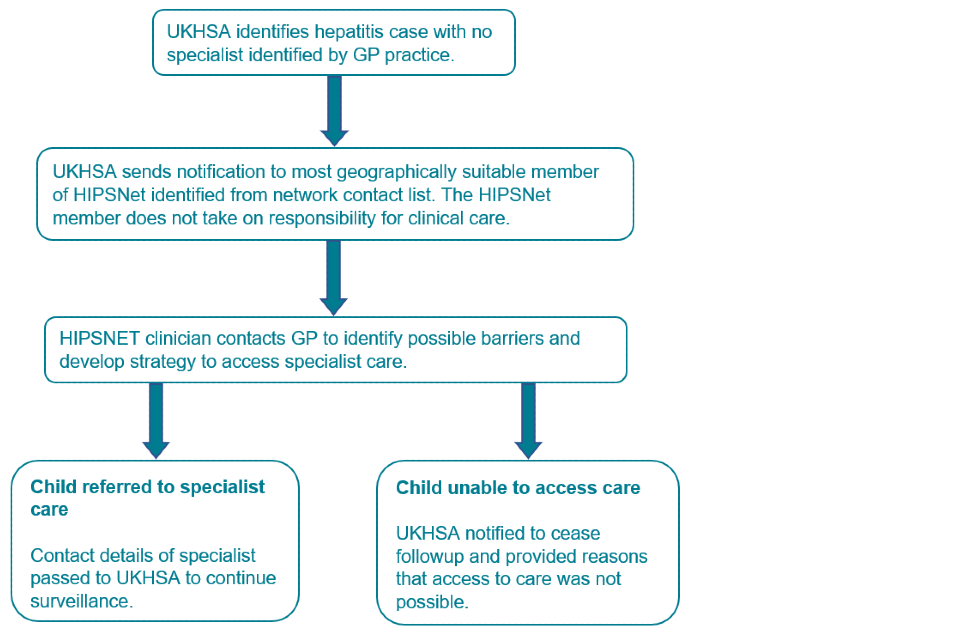
Accessible text equivalent of flowchart 3c
UKHSA identifies hepatitis case with no specialist identified by GP practice.
UKHSA sends notification to most geographically suitable member of HIPSNet identified from network contact list. The HIPSNet member does not take on responsibility for clinical care. HIPSNET clinician contacts GP to identify possible barriers and develop strategy to access specialist care. Child referred to specialist care
Contact details of specialist passed to UKHSA to continue surveillance. Child unable to access care.
UKHSA notified to cease follow-up and provided reasons that access to care was not possible.
Appendix 4.4. Privacy notice
Details on how we protect, store and how long we keep your information, along with your rights and UKHSA’s legal basis to use your this information can be found on UKHSA’s privacy information page.
