HPR volume 14 issue 22: news (24 November)
Updated 23 December 2020
ESPAUR annual report in summary
The annual English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR) report, published by PHE on 18 November, provides data on antibiotic use and resistance in the financial year 2018 to 2019.
Some findings of the seventh report are discussed below.
Overall rise in antibiotic resistance
There was a 32% increase in the estimated number of antibiotic resistant bloodstream infections from key bacterial species between 2015 and 2019.
One in five people with a key bacterial bloodstream infection had a bacteraemia resistant to at least one key antibiotic. This suggests that an estimated 65,162 antibiotic-resistant severe infections occurred in 2019; equivalent to 178 new antibiotic-resistant infections per day. (See following news items for reports on 2 pathogen-specific surveillance systems.)
PHE worked closely with the Department of Health and Social Care (DHSC) to add ‘Acquired-carbapenemase-producing Gram-negative bacteria’ to the list of causative agents under Schedule 2 of the Health Protection (Notifications) Regulations 2010. The change in legislation is effective from 1 October 2020.
Overall antibiotic usage falling
Antibiotic consumption has been falling since the peak in 2014. From 2015 to 2019, the total use decreased from 19.4 to 17.9 defined daily doses (DDDs) per 1,000 inhabitants per day.
General practices accounted for 71% of all antibiotics prescribed. Although consumption in this setting as well as in the dental sector has continued to decrease (by 12.2% and 19.5% respectively), hospital and other community settings continue to see increases in usage.
Antibiotic use continues to climb in secondary care (3.5% increase from 2015 to 2019 by hospital admissions), albeit at a slower rate of increase than the previous 5 years (6% from 2010 to 2014).
Antimicrobial stewardship
More than 2,400 UK healthcare workers participated in a survey on knowledge, attitudes and behaviour towards antibiotic use and resistance. The majority of participants correctly answered that antibiotics are not effective against viruses (97%), they have associated side effects (97%), unnecessary use makes antibiotics ineffective (97%) and healthy people can carry antibiotic resistant bacteria (90%). However, fewer respondents correctly answered that using antibiotics increases a patient’s risk of antimicrobial resistant infection (80%) or that resistant bacteria can spread from person to person (78%).
Medical doctors, as a group, had the highest percentage of their number answering all 7 questions correctly (80%) followed by pharmacists (74%) and dentists (68%).
The majority of healthcare workers agreed they have easy access to the guidelines they need to manage infections (80%). However, fewer agree that they have easy access to the materials they need to provide advice (68%) and even fewer have good opportunities to provide that advice (62%).
Of the 699 respondents that prescribed, dispensed or administered antibiotics at least once a week, only 21% had given out resources on antibiotic use at least once in the previous week. A higher proportion (61%) of respondents gave out advice at least once in the previous week.
The top 3 barriers to providing advice or resources on responsible antibiotic use were lack of resources (19%), insufficient time (11%) and the patient being uninterested in the information (7%).
GRASP annual report in summary
PHE has published its annual Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP) report, presenting the latest data on antimicrobial resistance in Neisseria gonorrhoeae in England and Wales.
Between 2018 and 2019, gonococcal isolates collected through the GRASP sentinel surveillance system showed that there was a decrease in reduced susceptibility to ceftriaxone, the current first-line therapy. The proportion of isolates with reduced susceptibility to ceftriaxone (minimum inhibitory concentration (MIC) >0.03 mg/L) decreased from 7.1% in 2018 to 2.9% in 2019; this contrasts with the steady rise in the proportion of isolates with reduced susceptibility observed from 2013 to 2018 (0.3% to 7.1%).
Outside of the sentinel surveillance system, 3 cases of ceftriaxone resistance (MIC >0.125 mg/L) were confirmed by the PHE Antimicrobial Resistance in STIs (AMRSTI) national reference laboratory on direct referral in 2019. These cases were unrelated, although all were associated with travel from the Asia Pacific region. There was no evidence of acquisition or transmission within the UK and all cases were successfully treated with 1 g ceftriaxone.
Between 2018 and 2019, testing of gonococcal isolates collected through GRASP also showed:
- azithromycin resistance decreased marginally from 9.8% to 9.3%
- cefixime resistance decreased from 2.2% to 0.8%
- ciprofloxacin resistance increased from 39.8% to 42.7%
- penicillin resistance increased from 12.4% to 17.9%
- tetracycline resistance increased from 52.8% to 62.9%
The marginal decline in the proportion of isolates resistant to azithromycin (MIC >0.5 mg/L) in 2019 and, most notably, the substantial reduction in resistance to cefixime (MIC >0.125 mg/L) represents a change in the trend in antimicrobial resistance observed from 2016 to 2018. As in previous years, no spectinomycin resistance (MIC >64 mg/L) was detected in 2019. Ciprofloxacin and penicillin resistance have been rising since 2016 and 2017 respectively, while sharp annual increases in tetracycline resistance have been reported since 2015.
Antimicrobial susceptibility testing for the sentinel surveillance system included gentamicin for the first time in 2019. While no resistance breakpoint currently exists for gentamicin, the modal MIC was low (4 mg/L).
Prescribing data demonstrated excellent adherence to the updated UK first-line treatment guideline, with 94.6% of individuals receiving the recommended ceftriaxone 1 g monotherapy, in 2019.
The effectiveness of first-line treatment for gonorrhoea continues to be threatened by the development of antimicrobial resistance. However, the decline in reduced ceftriaxone susceptibility, together with decreasing cefixime resistance and stable rates of azithromycin resistance, are encouraging developments.
Sexual health services should report cases of suspected treatment failure to PHE via the online HIV and STI web portal.
New pilot report on drug-resistant Mycoplasma genitalium
PHE’s recently-published, first pilot report on Mycoplasma genitalium Antimicrobial Resistance Surveillance (MARS) presents latest data on antimicrobial resistance in M. genitalium from specimens collected from sentinel sexual health clinics in England.
The pilot collated data from all consecutive M. genitalium specimens collected from 17 clinics between January and March 2019. Clinics performed M. genitalium diagnostic testing for those presenting with non-gonococcal urethritis or pelvic inflammatory disease, and their current sex partners. Specimens sent to the PHE’s Antimicrobial Resistance in STIs (AMRSTI) national reference laboratory were then tested for molecular markers predictive of macrolide and fluoroquinolone resistance in the M. genitalium 23S rRNA and parC gene, respectively.
Among 352 individuals included in the MARS pilot, 283 (80%) were symptomatic. 254 (72%) were men, 188 (74%) of whom identified as heterosexual. 144 (41%) were of White ethnicity and 150 (43%) were aged 25 to 34 years old. Of the corresponding 352 specimens submitted, 249 (71%) were successfully tested for macrolide resistance and, among these, 173 (69%) were predicted to be resistant. Most specimens from women (67%), heterosexual men (66%) – and most notably, from gay, bisexual and other men who have sex with men (MSM) (85%) – displayed macrolide resistance.
Macrolide resistance mutations were frequent among specimens from people of White (66%) and Black or Black British (72%) ethnicity, and were more common among specimens from individuals who had a previous sexually transmitted infection (STI) in the past year (84%) than those who did not (66%). A total of 251 (71%) specimens were successfully tested for fluoroquinolone resistance and 21 (8%) were predicted to be resistant. Predicted resistance to both macrolides and fluoroquinolones was detected in 12 (5%) of 237 specimens.
Azithromycin was prescribed either alone or as a component of first treatment for 195 individuals. Among those, 21 (11%) failed treatment, as indicated by a positive test-of-cure, all of whom had specimens which had mutations associated with macrolide resistance. Moxifloxacin was prescribed either alone or as a component of first treatment for 139 individuals, of which 4 (3%) failed treatment. Among those, 3 (75%) had specimens which had a mutation associated with fluoroquinolone resistance.
MARS is a scalable means of continued M. genitalium surveillance and will provide a rich resource for informing future updates to management guidelines in the UK.
Sexual health services should report cases of suspected treatment failure to second-line therapeutics to PHE via the online HIV and STI web portal.
Annual review of infections in UK blood, tissue and organ donors
The joint NHS Blood and Transplant and PHE Epidemiology Unit has published its annual summary of latest data and research activity Safe Supplies Annual Review on the GOV.UK website. The full report is available on the NHS Blood and Transplant website.
The joint unit is responsible for the surveillance systems which report on infections in blood, tissue, cell and organ donations across the UK. This year’s summary report, titled Safe Supplies 2019: data in Context, presents data up to 2019. Where possible, infections in donor and broader general populations is presented in order to provide context for the findings. The review covers the continued monitoring of the effects of changes to donor selection with updated residual risk estimates among blood donations, a summary of markers of infections detected in blood, tissue and organ donors and reported transfusion transmitted infections.
In November 2017, the English, Welsh and Scottish blood services changed their donor selection criteria for individuals with sexual partners at increased risk of infection from a 12-month to a 3-month deferral since the last time they had sex; Northern Ireland changed in 2020. Increased risk partners included men who have sex with men (MSM) and commercial sex workers. Surveillance post-change suggests reducing the sexual deferral period has had no major impact on blood safety with no increase in viral infections. In 2019, work progressed to explore if a more individualised risk assessment approach to blood donor selection policy is possible whilst ensuring the safe supply of blood.
The Epidemiology Unit’s annual review reports that over 1.8 million blood donations were screened across the UK in 2019, with 206 (0.01%) donations confirmed positive for either hepatitis B virus, hepatitis C virus, HIV, HTLV or Treponemal antibody (syphilis) and discarded. The majority of these were detected in donations from new donors (173, 84%) and these were mostly either past or chronic infections in the donor.
The residual risk of releasing an HBV, HCV or HIV infectious blood donation was estimated for 2017 to 2019 as less than 1 in a million. HBV risk remained stable until 2018 when the risk increased due to 7 recent infections in repeat donors, however these infections were not associated with the 3-month deferral and this increase was not sustained in 2019. Since 2012 the HIV and hepatitis C virus risk declined. Residual risk estimates are not the transmission risk and observed TTIs are lower in practice.
A further 552 blood donations were positive for hepatitis E virus and discarded. HEV rates have increased, there is no specific deferral as the main risk is dietary in the UK.
During 2019, one transfusion transmitted hepatitis E infection was confirmed, the second in a screened blood donation and in addition a transmission of hepatitis B was reported to the Serious Hazards of Transfusion haemovigilance scheme (SHOT) as probable. In 2019, there were no proven bacterial transmissions reported to SHOT.
Another role of the unit is in horizon scanning for infectious diseases that could potentially affect the safety of the UK blood supply, particularly new and emerging infections, in conjunction with colleagues across PHE and blood services across the world. Data contribute to risk assessments and may result in additional tests or changes to donor selection criteria being implemented – for example, additional testing for West Nile virus in donors returning from affected areas was extended to Cyprus and Var region in the south of France in 2019.
Tissue, cell and organ donations
In recent years there has been a decrease in the number of living donors donating bone due to good stock levels. In 2019, no donors tested positive. Deceased tissue donors have the highest rates of positivity which may reflect their age; 4 were positive for syphilis, 6 for HBV and 4 HCV. During 2019, 2 cord blood donors tested positive for syphilis – antenatal screening will generally select out women positive for HBV, HIV and syphilis.
NHSBT is also responsible for deceased organ donation across the UK. Some deaths in the UK are in circumstances where organ donation is possible, but donors or families must agree. Among 2,196 consented, deceased potential donors, 1,653 (75%) became actual donors where at least 1 organ was retrieved for transplantation and for 1,529 (92%) an organ was utilised. Organ donation can save and transform lives, and markers of infection are not necessarily barriers for transplantation. Among those who donated, 19 were screen reactive for markers of HBV, HCV, HTLV or HEV.
Information about blood donation is available at: www.blood.co.uk or by calling 0300 123 2323. Those wishing to donate organs may join the organ donor register at: www.organdonation.nhs.uk.
Requests for further information about NHSBT data should be directed to: [email protected]
EVD in Equateur province, DRC: outbreak declared over
The Democratic Republic of Congo’s (DRC) 11th Ebola virus disease (EVD) outbreak was declared over on 18 November by the World Health Organization (WHO), after 170 days. This represents the first time since August 2018 that the DRC government is not responding to an active outbreak of EVD.
Since the initial cases were announced on 1 June 2020, 130 cases (119 confirmed and 11 probable), including 55 fatalities, have been reported. The last confirmed case was discharged as cured in early October. Two probable cases were reported in late October but reflected infections acquired in July and August that were validated retrospectively.
The Equateur outbreak 2020 has been regarded as a ‘slow-burn’ outbreak, with cases reported in relatively low numbers over a 4-month period, as illustrated in the epidemic curve below. (The figure shows new (blue bars) and cumulative-total cases (red line), by week, between June and November 2020.)
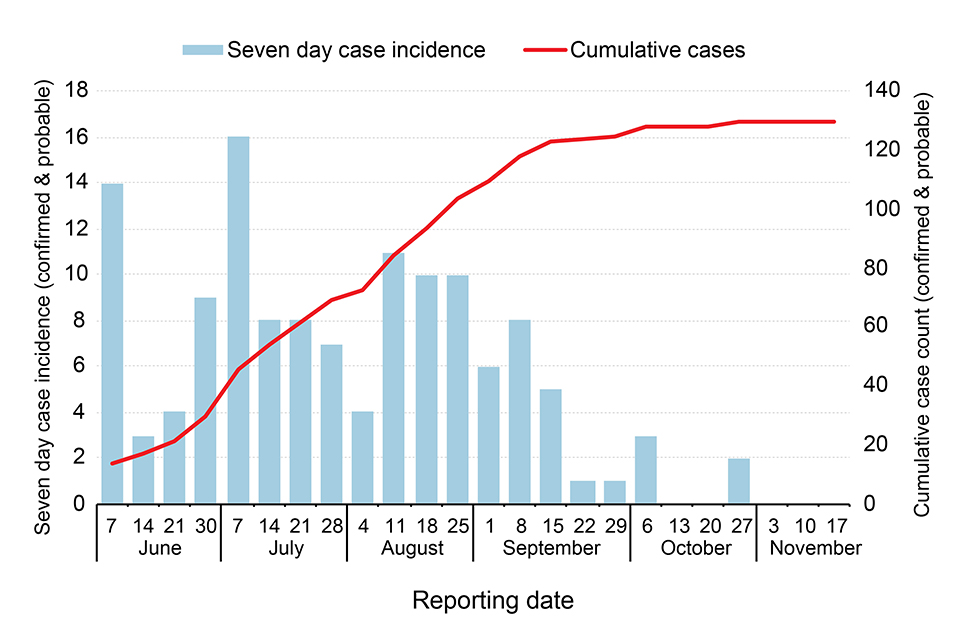
This outbreak occurred over a wide geographical expanse in Equateur province, in northwest DRC, with cases reported in 13 of the province’s 18 health zones. The wide spread of this outbreak stretched the ability and capacity of responders in the province. Many cases and contacts were in difficult to reach, very remote rural settings, accessible only by small power boats, delaying time-sensitive actions such as case isolation, contact tracing and the provision of care.
New variant associated with Equateur outbreak
Preliminary whole genome sequence analysis has suggested that 2 distinct variants of Ebola virus have circulated concurrently in this outbreak. Based on analysis carried out in August 2020 of 34 samples, 33 samples from multiple health zones were attributed to a new variant observed only in this outbreak referred to as the dominant 2020 Equateur variant. One sample differed from the dominant variant and was most closely related to virus associated with the 2018 EVD outbreak in Equateur. This case is likely the result of transmission initiated from a persistently infected source, most likely a survivor from the 2018 outbreak, or possibly from exposure to the same zoonotic reservoir that is believed to have initiated the 2018 outbreak. Investigations are ongoing to determine the relative role of both these variants on the outbreak.
Although the outbreak has been officially declared over by the WHO, DRC has begun a 90-day period of enhanced surveillance to ensure the rapid detection of any further cases that might arise as a result of a missed transmission chain, reintroduction from an animal reservoir, or re-emergence of virus that has persisted in a survivor.
The largest EVD outbreak to have occurred in DRC was in North Kivu, South Kivu and Ituri provinces, Eastern DRC, between 2018 and 2020. In total 3,481 cases and 2,299 deaths were recorded during that 10th DRC outbreak, making it the second largest Ebola outbreak recorded to date after the West African outbreak in 2014 to 2016.
In addition to the management of this latest Ebola outbreak, health authorities, humanitarian organisations and charities in the country have been responding to other, concurrent infectious disease incidents such as COVID-19, measles, monkeypox, circulating vaccine-derived polio virus and plague, as well as the tail-end of the10th Ebola outbreak.
Infection reports
Common animal-associated infections (England and Wales): first and second quarters 2020.
Vaccine coverage report
Impact of COVID-19 childhood vaccination counts up to week 45 (England).
