HPR volume 9 issue 26: news (24 July)
Updated 29 December 2015
1. Cyclospora outbreak related to travel to Mexico
As of 24 July, 24 cases of cyclospora infection have been reported in England and Scotland in June and July 2015, of which 21 were associated with travel to Mexico. No cases have been reported in Wales or Northern Ireland to date. Cases have been to various hotels and resorts on the Riviera Maya coast of Mexico, which includes Cancun, Playa del Carmen and Sian Ka’an, suggesting the source may be a food product that was distributed to several hotels.
Most cyclospora cases in England and Wales in recent years have been reported between weeks 23 and 33 (in June and July) so the current excess is not unusual at this time of the year. However in previous years on average one case per year has been associated with travel to Mexico, so the recent excess of cases linked to Mexico is unusual. There is currently an outbreak of cyclospora in Texas with over 182 cases, and large outbreaks in Texas in 2013 and 2014 were associated with Mexican salad products [1,2].
Cyclospora cayetanensis is a coccidian protozoan parasite that infects humans and other primates. Infection is characterised by diarrhoea, abdominal cramping, nausea, flatulence, anorexia, fatigue, low-grade fever, and weight loss and is commonly derived from food or water contaminated by human faeces [3,4]. The oocysts of this organism are not infectious for around 10 days after they are passed in faeces and person-to-person transmission does not occur. The foods previously involved include soft fruits such as raspberries and salad products such as coriander, basil and lettuce.
There may be substantial under-ascertainment and reporting of cyclospora cases, because not all patients are tested for cyclospora and not all positives are reported by laboratories. In addition, these organisms can be difficult to spot and recognise in unstained wet films or concentrates. Faecal samples can be examined using a wet prep, and if structures resembling cyclospora are observed, the slide can be viewed under UV light as the parasite autofluoresces or stained using Modified ZN staining on fixed films [5].
In view of the ongoing outbreak we recommend that patients returning from Mexico with diarrhoea are tested for cyclospora. Cases in England should be reported to the Public Health England (PHE) local health protection team, and positive samples referred to the Cryptosporidium Reference Unit in Swansea for confirmation and typing.
Health advice for travellers to Mexico, including advice on food and water hygiene, can be found on the NaTHNaC website.
1.1 References
- Abanyie F, Harvey RR, Harris JR, Wiegand RE, Gaul L, Desvignes-Kendrick M, Irvin K, Williams I, Hall RL, Herwaldt B et al (2015). 2013 multistate outbreaks of Cyclospora cayetanensis infections associated with fresh produce: focus on the Texas investigations. Epidemiology and Infection 2015: 1-8.
- Centers for Disease Control and Prevention (2013). Outbreaks of cyclosporiasis – United States, June-August 2013. Morbidity and Mortality Weekly Report (MMWR) 62(43): 862.
- Ortega YR, Sanchez R (2010). Update on Cyclospora cayetanensis, a food-borne and waterborne parasite. Clinical Microbiology Reviews 23(1): 218-234.
- Chacin-Bonilla L (2010). Epidemiology of Cyclospora cayetanensis: a review focusing in endemic areas. Acta tropica 115(3): 181-193.
- PHE-SMI B31. UK Standards for Microbiology Investigations: Investigation of specimens other than blood for parasites.
2. Ebola virus disease: international epidemiological summary (at 19 July 2015)
The Ebola Virus Disease (EVD) outbreak in West Africa continues with cases reported in 2 countries this week. As of 19 July 2015, a total of 27,741 clinically compatible cases of EVD have been reported associated with this outbreak, 11,284 of which have died.
There were 26 confirmed cases of EVD reported in the past week: 22 in Guinea and 4 in Sierra Leone, compared to 13 in Guinea, 3 in Liberia and 14 in Sierra Leone in the previous week.
The main foci of transmission remain within Conakry and Freetown, the capital cities of Guinea and Sierra Leone respectively, for the second week in a row.
All but two of the 26 cases arose among registered contacts of previous EVD cases indicating improvements in contact ascertainment and monitoring in both countries.
Three new health care worker infections were recorded, 2 in Guinea and 1 in Sierra Leone.
No new cases were reported in Liberia where 6 cases have been confirmed since 29 June 2015. Investigations are ongoing into the source of this outbreak
On 20 July, Italy was declared EVD free after the completion of 42 days since their only EVD case tested negative and was discharged from hospital.
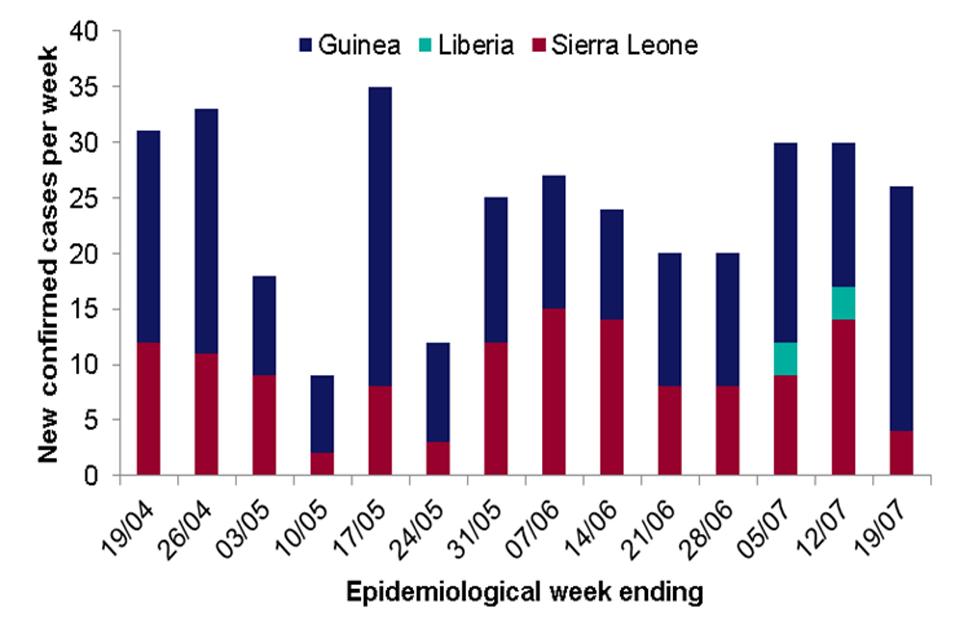
Number of new confirmed cases reported per week (19 April to 19 July 2015) in affected countries in West Africa. (Data Source: WHO Ebola Situation Report 22 July 2015)
Further information on the epidemiological situation can be found in PHE’s weekly Ebola epidemiological update and from the Ebola Outbreak Distribution Map.
3. Transfusion transmitted infections (UK): 2014
A description of the possible transfusion-transmitted infection incidents investigated by the United Kingdom (UK) Blood Services in 2014 has been published in the Serious Hazards of Transfusion (SHOT) annual report [1].
The risk of a screened component transmitting hepatitis B virus (HBV), hepatitis C virus (HCV) or human immunodeficiency virus (HIV) in the UK is very low [2]. Nevertheless, to maintain haemovigilance, investigations are performed if a recipient is suspected to have been infected via transfusion.
UK Blood Service investigations in 2014 have confirmed that there were:
-
no proven bacterial transfusion-transmissions reported in 2014
-
2 near miss bacterial incidents
-
1 transfusion-transmitted hepatitis E virus (HEV) incident following a transfusion in 2014 affecting 2 recipients
The risk of bacterial transmission is not completely abolished by bacterial screening of platelets. Alerting the Blood Service immediately of significant adverse reactions including those suspected of being the result of bacterial contamination of a component in order allows associated packs to be recalled if necessary.
Suspected viral transmissions should also be reported to the blood services who can advise on the information required and how to proceed. The HEV incident in 2014 was reported by one of the health protection teams to the Blood Service for investigation. Blood donations in the UK are not currently screened for HEV. The Advisory Committee on the Safety of Blood, Tissues and Organs (SaBTO) has set up a working group to consider the risk of hepatitis E transmission via blood in the UK and what action, if any, should be taken. Recommendations from this work are likely to be in the public domain by the Autumn of 2015.
See the SHOT annual report [1] for a fuller description of incidents investigated in 2014 with commentary on CMV, cumulative data by transfusion year and advice on what to do if you suspect a transfusion transmitted infection incident has occurred.
For further information please contact the NHSBT/PHE Epidemiology Unit at: [email protected].
3.1 References
- NHSBT (2015). SHOT Annual Report 2014.
- NHSBT/PHE (September 2014). Safe supplies: reflecting on the population 2013 annual review.
4. One Health Report on human and animal antibiotic use, sales and resistance (UK): 2013
The joint One Health report – presenting data on antimicrobial usage and bacterial resistance in selected human and animal pathogens in the UK – was published by PHE and the Veterinary Medicines Directorate on 22 July 2015 [1].
The One Health report brings together the most recently available UK data on antibiotic resistance in key bacteria that are common to animals and humans and, details on the amount of antibiotics sold for animal health and welfare and antibiotics prescribed to humans, with the following aims:
-
to encourage further joint working between the human and animal sectors
-
to identify the emerging and current antibiotic resistance threats in 3 key bacteria in humans and animals
-
to identify differences in surveillance methodology and data gaps that limit our ability to compare trends between the 2 fields, both within the UK and across Europe
-
to evaluate available data from humans and animals side by side and begin to assess the relationship between antibiotic sales, use and resistance across the 2 sectors
-
to develop recommendations to improve the surveillance of antibiotic use and resistance in humans and animals
The bacteria selected for this report are based on the following: bacteria that are transmitted through the food-borne route (salmonella and campylobacter) and Escherichia Coli (E. Coli), an important organism that lives in the gut of both humans and animals and can cause opportunistic and invasive disease in all species.
There are many caveats surrounding interpretation of the data presented in the report and in some cases the methods of data collection vary to such an extent that they cannot be meaningfully compared. This highlights the joint responsibility of the human and animal sectors in tackling antimicrobial resistance (AMR) and the importance of strengthened collaboration between them.
4.1 Escherichia Coli
In 2013, 35,716 bloodstream infections in people due to E. Coli were reported, making it the commonest cause of bloodstream infection in the UK. Antibiotic resistance results were available for more than 70% of these infections. Third-generation cephalosporin (cefotaxime and/or ceftazidime) resistance was reported in 10%, ciprofloxacin resistance in 18%, piperacillin-tazobactam resistance in 9% and carbapenem resistance in less than 1%. These are important antibiotics for the treatment of this infection.
In 2013, clinical surveillance yielded 3,320,807 isolates of E. Coli from all livestock groups. Resistance to the third-generation cephalosporins cefotaxime and ceftazidime was seen in 11% and 6%, respectively; no antibiotic susceptibility testing (AST) for ciprofloxacin, piperacillin-tazobactam or carbapenems was performed. Enrofloxacin resistance was 6%; ciprofloxacin is an active metabolite of enrofloxacin, an antibiotic authorised solely for veterinary use. EU harmonised surveillance from pigs reported <1% of cefotaxime and ciprofloxacin resistance; carbapenems and piperacillin-tazobactam were not tested.
4.2 Campylobacter
Campylobacter gastroenteritis was the most common human-acquired bacterial zoonosis, with 66,575 cases reported in 2013. The majority of infections are self-limiting and do not require antibiotic treatment. However, in cases of invasive infection, severe disease or when individuals are immunocompromised, antibiotic treatment is required. Antibiotic resistance results were available for approximately 45% of bacterial isolates. Ciprofloxacin resistance was reported in 42% and erythromycin resistance in 2.5%. EU-harmonised surveillance of AMR in healthy pigs at slaughter yielded 141 Campylobacter Coli (C. Coli) isolates with 13% ciprofloxacin resistance and 28% erythromycin resistance. Similar surveillance performed in broiler chickens found 31% ciprofloxacin resistance in 61 C. Jejuni isolates, 55% resistance in 33 C. Coli and 3% erythromycin resistance in 33 C. Coli.
4.3 Salmonella
As with campylobacter, salmonella infections are frequently self-limiting and require no treatment; however, antibiotics may be necessary in severe cases. In 2013, 8,459 human cases of non-typhoidal salmonella infections were reported in the UK through routine laboratory surveillance, with more than 70% referred to the reference laboratories for speciation and antibiotic resistance testing. Resistance to cefotaxime and ciprofloxacin was noted in 2% and 16% of tested isolates, respectively.
Salmonella species vary depending on the animal species from which they are isolated. Clinical and statutory surveillance of salmonella in animals showed very different resistance profiles across animal species: antibiotic resistance was uncommon in salmonella species from sheep or cattle but more frequent in salmonella species from pigs or turkeys. EU-harmonised surveillance was performed in healthy broilers, layers, turkeys and pigs in 2013. Cefotaxime resistance was rare: in salmonella isolated from pigs it was 2% and was not detected in other animals. Ciprofloxacin resistance was not detected in 2,2761,834 isolates from clinical surveillance. Cefotaxime and ciprofloxacin resistance were rare.
4.4 Antibiotic prescriptions and sales in humans and animals
In 2013, total antibiotics dispensed to humans through prescriptions was 531.2 tonnes and total sales for animal use comprised 418.7 tonnes, ie of the total antibiotic use that was measurable in the UK, humans used 56% of total antibiotic tonnes used. The most frequently used antibiotics in humans were penicillins (64%) and tetracyclines (10%). Antibiotics sold for animal use were most frequently tetracyclines (43.5%) and penicillins (21.7%). Four antibiotic groups are defined by the World Health Organization as critically important for human use: macrolides, quinolones, cephalosporins and glycopeptides. More of these antibiotics are used in humans than animals.
The One Health report is an important first step in building the data required to contain antibiotic resistance and to develop coordinated surveillance activities regarding antibiotic use and resistance in human and animal health across the UK and Europe. For the 3 bacteria in this report, significant resistance is identified from human and animal surveillance across a wide range of antibiotics. The aim is for the approach adopted in the report to be enhanced in public and professional activities to develop cross-sectoral understanding and improved working in the future.
