Diphtheria anti-toxin: clinical guidance (issued May 2022)
Updated 7 November 2024
Applies to England
Diphtheria anti-toxin
Before the introduction of diphtheria anti-toxin (DAT), mortality rates for clinical diphtheria frequently exceeded 50%. One clinical trial found mortality of 3.3% in patients treated with DAT compared with 12.2% in untreated patients[1].
Early treatment with DAT is critical as protection is inversely related to duration of clinical illness preceding administration.
Treatment for probable and confirmed diphtheria cases should be discussed with an infectious diseases clinician – if the patient has a classic respiratory diphtheria presentation, then there is no need to wait for laboratory confirmation of a toxigenic strain before administering DAT.
DAT is based on a horse serum and anaphylaxis occurs more commonly than with human immunoglobulin products. However, from our experience in England of treating patients with DAT, anaphylaxis is very rare.
A single diphtheria anti-toxin (equine) product is currently being supplied in the UK.
Diphtheria anti-toxin (DAT) manufactured by Butantan Institute (Instituo Butanan) in 10ml ampoules containing 10,000 IU per ampoule (at least 1,000 IU DAT per ml).
Indications
DAT indications are for treatment of:
- suspected diphtheria cases*
- confirmed infections, where clinically appropriate, due to toxigenic Corynebacteria ulcerans or C. diphtheria
* as defined in national guidelines
Notes
Diphtheria anti-toxin should not be used for diphtheria prophylaxis.
Contacts should be given diphtheria-containing vaccine and antibiotic prophylaxis in line with national guidelines.
Treatment should proceed as soon as possible, as the effectiveness of DAT is strongly related to time since disease onset.
Dosage
Dosage for diphtheria anti-toxin is determined by the severity and duration of the disease as shown in the table below. The dose is the same for adults and children, and the number of ampoules required is also shown.
| Type of diphtheria | Dosage adults and children | Number of ampoules (10,000 IU/ampoule) |
|---|---|---|
| Severe diphtheria, for example, extensive membrane or severe oedema (‘bull neck’) | 100,000 IU | 10 |
| Laryngeal or pharyngeal or nasopharyngeal disease of more than 48 hours | 100,000 IU | 10 |
| Laryngeal or pharyngeal or nasopharyngeal disease of less than 48 hours | 70,000 IU | 7 |
| Skin lesions | 40,000 IU | 4 |
The World Health Organization (WHO)[2] states that anti-toxin is of limited value in cutaneous disease. In most cutaneous infections, large-scale toxin absorption is unlikely and therefore the risk of giving anti-toxin is usually considered substantially greater than any benefit. Nevertheless, if the cutaneous ulcer is sufficiently large (that is, more than 2cm squared) and membranous, then anti-toxin may be justified.
Note: this guidance will differ from the dosage instructions in the Summary of Product Characteristics (SmPC) distributed with the product. In this instance, this guidance document should be followed.
Please read the Administration section carefully prior to giving anti-toxin.
Administration
Precautions for administration
Prior to administration, a detailed history should be taken including:
- previous administration of equine-derived anti-toxin or immunoglobulins
- documented or known animal allergy, specifically equine (horse) allergy
Route
The intravenous (IV) route is the preferred route of administration of DAT, especially in severe cases.
The anti-toxin dose should be mixed in 250ml to 500ml of normal saline (that is, sodium chloride 0.9% injection) and administered slowly over 2 to 4 hours, closely monitoring for anaphylaxis.
Delivery of dose
-
Perform sensitivity tests if indicated, and desensitisation if necessary.
-
Give the entire treatment dose of anti-toxin IV in a single administration (except for series of injections needed for desensitisation), unless there is the onset of allergic response. Divided doses are not recommended as this increases risk of sensitivity reactions (see section on side effects).
However, if in exceptional circumstances, the full dose cannot be given in a single administration, for example, in a small child, the maximum interval between the 2 split doses should not exceed 24 hours. If a divided dose is delayed by 72 hours, sensitivity testing should be carried out. For any divided dose between 24 and 72 hours, administration should proceed with caution.
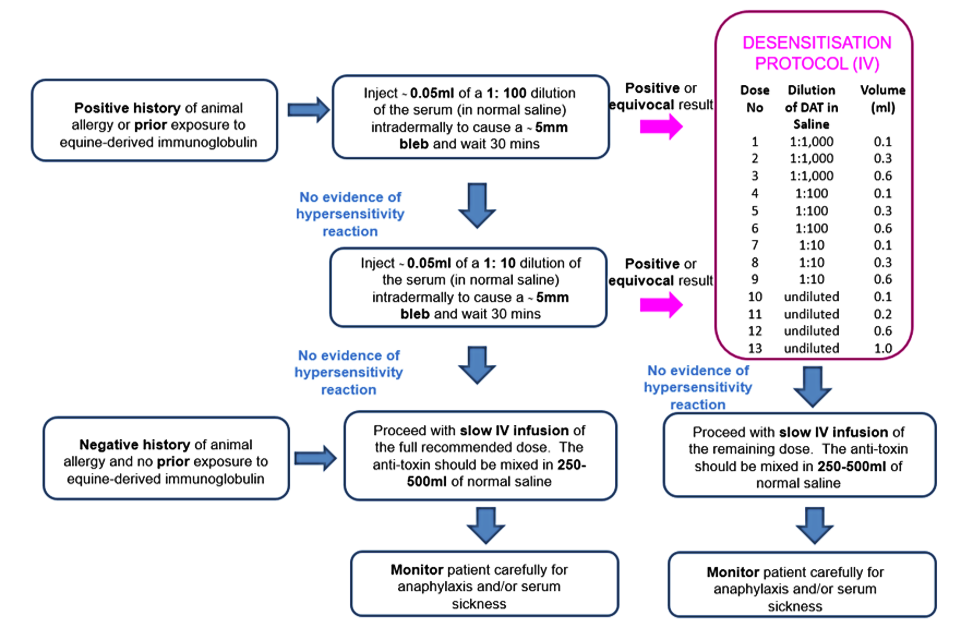
Figure 1. Summary of DAT administration according to patient history and sensitivity testing
See sensitivity testing section below for further details.
Sensitivity testing
In people with a negative history for animal allergy and no prior exposure to equine-derived immunoglobulin:
Do not perform sensitivity testing and proceed with a slow IV infusion of full recommended dose.
The anti-toxin should be mixed in 250ml to 500ml of normal saline and administered slowly with 10% of volume over the first 30 minutes, and the remainder over 2 to 4 hours, with close monitoring for anaphylaxis.
In patients with a positive history for animal allergy or prior exposure to equine-derived immunoglobulin suggesting increased risk:
1. Inject ~0.05ml of a 1:100 dilution of the serum (in normal saline) intradermally to cause a ~5mm bleb, and wait 30 minutes.
2. If no evidence of hypersensitivity reaction, repeat intradermal injection using a 1:10 dilution of the serum (in normal saline) and wait 30 minutes.
3. If no evidence of hypersensitivity reaction (erythema, itch), proceed with a slow IV infusion of full recommended dose. The anti-toxin should be mixed in 250ml to 500ml of normal saline and administered slowly with 10% of volume over the first 30 minutes, and the remainder over 2 to 4 hours, with close monitoring for anaphylaxis.
4. If intradermal testing is positive or equivocal, then the schedule of desensitisation should be followed. To dilute for intradermal testing, make serial dilutions as follows (see also Densensitisation to DAT – intravenous route table):
- 0.1ml of serum + 0.9ml normal saline = 1:10 dilution
- 0.1ml of 1:10 dilution + 0.9ml normal saline = 1:100 dilution
Recent use of an antihistamine (in last 48 to 72 hours) – including antihistamines present in over-the-counter preparations such as cough remedies – may interfere with the intradermal test. In this case, either seek the advice of a specialist in allergy to undertake screening using a positive (histamine) and negative (saline) control, or follow the desensitisation protocol for someone with a positive intradermal test.
5. Monitor the patient carefully during treatment and ensure facilities for treating anaphylaxis (including 1:1,000 adrenaline for injection) are readily available.
Desensitisation to DAT
Patients with positive sensitivity testing to DAT or with a previous history of adverse reaction to DAT administration (even with a negative or equivocal intradermal test) should undergo desensitization, as shown in the table below. The IV route is considered safer because it offers better control[3].
Desensitization to DAT – intravenous route
| Dose number* | Dilution of DAT in normal saline | Amount of injection (ml) |
|---|---|---|
| 1 | 1:1,000** | 0.1 |
| 2 | 1:1,000 | 0.3 |
| 3 | 1:1,000 | 0.6 |
| 4 | 1:100** | 0.1 |
| 5 | 1:100 | 0.3 |
| 6 | 1:100 | 0.6 |
| 7 | 1:10** | 0.1 |
| 8 | 1:10 | 0.3 |
| 9 | 1:10 | 0.6 |
| 10 | undiluted | 0.1 |
| 11 | undiluted | 0.2 |
| 12 | undiluted | 0.6 |
| 13 | undiluted | 1.0 |
*Administer at 15 minute intervals
**1 ml (anti-toxin) + 9.0 ml of saline (sodium chloride 0.9% injection) = 1:10 dilution
1 ml (1:10 dilution) + 9.0 ml of saline = 1:100 dilution
0.1 ml (1:10 dilution) + 9.9 ml saline = 1:1,000 dilution
1 ml (1:100 dilution) + 9 ml saline = 1:1,000 dilution
The protection from anaphylaxis afforded by giving DAT according to this desensitisation protocol requires that no interruption occur in the sequence of administration of doses. Iif an interruption occurs the protection is lost.
If no hypersensitivity reaction occurs, administer remaining quantity of anti-toxin as above.
Antimicrobial therapy
Appropriate antimicrobial agents in full therapeutic dosages should be started in line with national guidelines.
Pregnancy
Diphtheria anti-toxin (equine) should be used cautiously during pregnancy.
Side effects
Administration of diphtheria anti-toxin may cause hypersensitivity reactions including anaphylaxis. Reactions occur in individuals previously sensitized to equine anti-toxin or horse proteins either through previous administration on in some other way.
Reactions to the anti-toxin may manifest as an anaphylactic reaction and/or serum sickness. This is why giving the required dose of anti-toxin in a single administration is recommended.
Anaphylaxis usually occurs within 1 to 2 hours of administration. The dose of adrenaline for anaphylaxis in teenagers and adults is 0.5ml of 1:1,000 adrenaline given intramuscularly, every 5 minutes.
In the event of severe anaphylaxis (no response to 2+ intramuscular (IM) injections of adrenaline), an adrenaline infusion may be needed – call for specialist support.
Serum sickness can occur in up to 5% of patients according to historic data, usually around 7 to 12 days after the first injection although accelerated reactions have been reported in patients who have previously received equine anti-toxin preparations, with onset within days or even hours.
Symptoms include more generalised erythema, urticaria, itching, and occasionally fever, pain and oedema of the joints and lymph nodes.
Treatment is supportive, with anti-inflammatory preparations and antihistamine to provide symptomatic relief – systemic steroids may be needed in more severe cases.
Storage
Store at 2ºC to 8ºC. Once the ampoule is opened, the preparation must be used immediately.
Acknowledgements
This guidance was prepared by: Gayatri Amirthalingam, Charlotte Gower, Meera Chand, Kevin Brown, Colin Brown, Mary Ramsay.
For queries relating to this document, please contact [email protected]
The authors gratefully acknowledge the expert review and advice received from: Dr Paul Turner, Honorary Consultant in Paediatric Allergy and Immunology Imperial College London.
References
1. ‘Diphtheria; other corynebacterial and coryneform infections’, in In Topley and Wilson’s principles of bacteriology, virology and immunity (chapter 60). 5th edition. Baltimore, MD: Williams and Wilkins, 1964, pp.1695 –98
2. WHO (1994) Diphtheria. Manual for the management and control of diphtheria in the European Region. WHO, Copenhagen
3. CDC (2016) Expanded Access Investigational New Drug (IND) Application Protocol: ‘Use of Diphtheria Antitoxin (DAT) for Suspected Diphtheria Cases’ IND Sponsor: Centers for Disease Control and Prevention (CDC) Protocol CDC IRB #4167
