MAVIS Hub 111
Published 24 July 2019
1. Product News
Tylan 200mg/ml Solution for Injection - Product defect recall alert
Norfenicol 300mg/ml Solution for Injection - Product defect recall alert
Alfaxan & Alfaxan Multidose 10mg/ml Solution for Injection - Product defect recall alert
Removal of the active substance Fenbendazole for use in pigeons from Schedule 6
Membership of the Veterinary Products Committee
Top ten imported products - Quarterly report 01 April to 30 June 2019
Suspension of Veterinary Medicines containing the excipient Diethanolamine (DEA)
2. Enforcement
Animal Medicine seizure notice: Sallins Farm
Animal medicines improvement notice: Mr Gilbert, Womblehill, Aberdeenshire
Animal medicines improvement notice: Charles CBD Therapies, South Tyneside
Animal medicines seizure notice: Mr Thompson
3. AMR (Antimicrobial Resistance)
UK’s approach to antimicrobial resistance gets UN recognition
3.1 Defra Antimicrobial Resistance Co-ordination (DARC) group
The DARC group met on 25 June 2019 to discuss the recent trends in antibiotic resistance (AMR) in bacteria of importance to human and animal health. The group received an update on antibiotic consumption data projects under development, as well as recent international collaborations. Topical presentations were given on the International Work of AMR with contributions from the Veterinary Medicines Directorate, Animal and Plant Health Agency, Food and Agriculture Organisation of The United Nations and World Organisation for Animal Health. Summary minutes of the meeting.
3.2 Sales Data and Antibiotic Resistance Surveillance Report
Data for the 2018 UK Veterinary Antibiotic Resistance and Sales Surveillance (UK-VARSS) Report are currently being collated for publication later in the year. The report collates UK data on antibiotic sales from Marketing Authorisation Holders and antibiotic resistance data from the VMD’s surveillance programmes.
UK-VARSS 2017 and previous reports are available on GOV.UK.
4. Guidance updates
Controlled drugs: recording, using, storing and disposal
As a vet or pharmacist, you must follow legal requirements when working with controlled drugs in veterinary medicine. Updated Currently Authorised Controlled Drugs table
Advertise veterinary medicines legally
How veterinary medicines can be advertised. Reviewed and updated to provide additional clarification.
Manufacturing and supplying veterinary medicines for animal feed
Guidance for manufacturers and suppliers of veterinary medicines for incorporation into animal feedingstuffs. Reviewed and amended; minor changes include term ‘Qualified Person’ to ‘Designated Person’
The cascade: prescribing unauthorised medicines
Guidance for prescribing vets on the use of the cascade. Reviewed and updated to add further clarification
Report a product defect: veterinary medicine
For a marketing authorisation holder only; how to report a defect with a veterinary medicine. List of recalled products updated
Veterinary Medicines Directorate: Events Calendar 2019
Events VMD will be attending, hosting or participating in to support and advise industry and stakeholders. Added joint VMD VPC event
Exemption from authorisation for medicines for small pet animals
How to comply with the exemption from the Veterinary Medicines Regulations that allow certain animal medicines to be sold without an authorisation. Updated listing for Fenbendazole
Known supply problems with animal medicines
Reported problems with the supply of an animal medicine and the date when the issue is expected to be resolved. Addition of known supply issue with vaccines for immunisation against Leptospira hardjo and Leptospira borgpetersenii.
5. Surveillance
Veterinary Medicines Pharmacovigilance Annual Review 2017: Summary
Residues of veterinary medicines in food: 2018
Residues of veterinary medicines in food: 2019
6. Stakeholder Engagement
VMD Survey of Controlled Drugs Disposal 2018 – Summary of responses
Joint VMD, VPC and Pharmaceutical Industry Information Event 2019
6.1 Feedback Results Company Meeting Questionnaires 2018/2019
During 2018/2019 a total of 38 company meetings were arranged to discuss on- going applications or potential applications. This is fewer than in previous years, although the number of teleconferences held did increase.
Only a third of questionnaires were returned, which although disappointing is perhaps not surprising given the increased activity around preparations for UK Exit from the EU. We do, however, encourage companies to complete the questionnaires as this not only supports the range of qualitative measures we have in place, but provides valuable information that we use to help us to improve our services.
We achieved our objective with the overall median score from feedback surveys for individual VMD company meetings to be at least good for 90% or more. All companies who submitted a return rated the VMD as at least good. Out of a score of 5 the usefulness of the VMD advice was rated on average at 4.7. The rating for VMD staff being well prepared was 4.9, and the scores for individual teams (Biologicals, efficacy, safety, quality and administrative) were rated between 4.75 and 5.
Should you wish to have a face to face meeting with us, please contact Chris Abbott [email protected] who can make the necessary arrangements. He can also arrange a teleconference if that is better suited to your needs.
When considering the need for a meeting please give careful consideration to the areas you would like to discuss. This will help us to identify the relevant VMD attendees. Please also provide an agenda and any supporting presentations at least five working days in advance of the meeting so that we can prepare and be ready to provide the required advice. 31st July 2019
7. Corporate news
7.1 VMD
Change to the VMD email address
VMD Published Standards 2019 to 2020: Monitoring performance
7.2 Staff Changes
The following staff changes took place during this quarter:
7.3 New staff
- Latisia Amulnathan joined the Inspections Administration team
- Maureen Bowden joined the Communications team
- Rezwana Ahmed joined the Licencing Administration team
7.4 Departing staff
- Mali Sabet
- June Mullins (Retired)
- Elizabeth Marier (2-year loan at Defra)
- Christine Paine (Retired)
7.5 VMD Organogram as at 01 June 2019
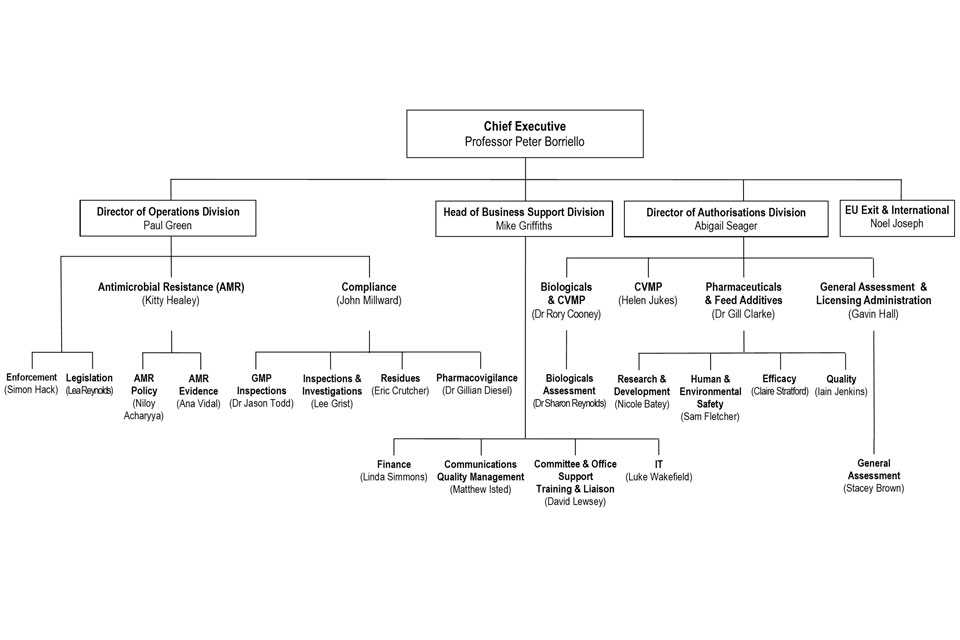
VMD Organogram as at June 2019
