MAVIS Newsletter edition 108
Published 1 November 2018
1. News
1.1 Brexit ‘no deal’ Technical Notices
Earlier this summer the Prime Minister set out plans to publish a series of technical notices outlining preparations for the UK leaving the EU without a deal.
The government does not want or expect a ‘no deal’ scenario and is committed to achieving a deal with the EU. However it is right that, as a responsible government, we continue to prepare for all scenarios, including the unlikely event of ‘no deal’.
Following the publication of initial notices in August, the third tranche of notices published at the end of September included three notices on veterinary medicines. The veterinary medicines technical notices can be accessed via the links below:
- Registration of veterinary medicines if there’s no Brexit deal
- Regulation of veterinary medicines if there’s no Brexit deal
- Accessing animal medicine IT systems if there’s no Brexit deal
In addition, we have published a presentation titled “‘no deal’ SI stakeholder engagement”.
1.2 MAVIS is changing and we need your help
What you need to know
We are changing MAVIS. From April 2019, we will replace the current MAVIS newsletter format with a new online MAVIS ‘Hub’. You will be able to access the ‘Hub’ via a quick link on our homepage.
What the ‘Hub’ will contain
The ‘Hub’ will contain regularly featured items as well as other useful links to frequently used areas of our website. We plan to refresh the featured information each quarter, based on the current publication cycle, and will include a ‘News Summary’ of items published over the previous quarter, grouped by subject.
Why we are doing this
We are doing this because the VMD’s 2018 Customer Satisfaction Survey results indicated that it was time for us to review the way we share information, to make it more accessible, relevant and searchable.
We need your help
We need your help to get this right. With a fresh new look in mind and starting from a blank page, we want to ensure the information we provide is useful and easy to access, so let us know:
• what information you would like on the ‘Hub’ and • which links to VMD pages you would like us to include, so it’s easier for you to find key things on our site?
Please send your comments and ideas to Carol Siwicka [email protected]
1.3 Customer Satisfaction Survey 2018
As previously published in April this year, the results of the 2018 Pharmaceutical Customer Satisfaction Survey were extremely pleasing. We appreciate the feedback we received and use this to help us improve the services we provide. Even though the scores reflected high levels of satisfaction, we have taken a more in depth review into those areas where common themes were emerging; or where the scores were slightly lower. The following table identifies these messages, and alongside we have noted the actions we are taking or where improvements had already been introduced just before the conclusion of the survey or shortly after. In some cases we have also included a reminder of long standing methods to help identify those dealing with the assessment of applications.
Validation
| Message/Theme | Actions/Improvements |
|---|---|
| Ease of identifying correct person to speak to. | Validator names provided in all email correspondence. |
| For general enquiries relating to validation or a particular procedure, a generic inbox will be created and advertised on related GOV.UK guidance pages. |
Joint Labelling
| Message/Theme | Actions/Improvements |
|---|---|
| Clarity of process & timescales | Guidance published on GOV.UK |
Product Literature Standard
| Message/Theme | Actions/Improvements |
|---|---|
| Clarity, consistency in its application, ease of navigation, and pragmatism in its use | Revised standard introduced shortly before the start of the survey. It is hoped that this will provide greater clarity and is more easily navigated. The HPRA were consulted and contributed to this revision. |
| Further links are being developed with the HPRA to help facilitate the process. |
Pharmaceutical & Feed Additives
| Message/Theme | Actions/Improvements |
|---|---|
| Consistency of approach between assessors & identifying the correct person to speak to | Assessor names provided in validation letters. |
| Changes in personnel reported in MAVIS and in Industry liaison meetings. | |
| Applications for new veterinary medicinal products are discussed at team meetings at quality, safety and efficacy level. | |
| Applications for new veterinary medicinal products are discussed by the Scientific Secretariat, a formal peer review meeting which includes VMD personnel and to which representatives from the Foods Standard Agency, the Environment Agency and Public Health England are invited to attend. |
Biologicals
| Message/Theme | Actions/Improvements |
|---|---|
| Consistency of approach between assessors & identifying the correct person to speak to | Assessor names provided in validation letters. |
| Changes in personnel reported in MAVIS and in Industry liaison meetings. | |
| Applications for new veterinary medicinal products are discussed at team level. | |
| Applications for new veterinary medicinal products are discussed by the Biologicals Committee, a formal peer review meeting. |
Pharmacovigilance
| Message/Theme | Actions/Improvements |
|---|---|
| Consistency of approach between assessors; relevance of questions & knowledge of staff responding to enquiries | Weekly adverse event assessor meetings are being held so that issues can be discussed and a more consistent approach followed by all assessors. |
| PSUR assessment training has been provided to all PSUR assessors. | |
| A desk instruction document has been drafted on how staff should respond to queries and how to deal with questions to which they are unsure how to respond. |
Communications
| Message/Theme | Actions/Improvements |
|---|---|
| Making people aware of new information in a timely fashion and ease of what you are looking for on the website. | RSS feed and email alert available to those who sign up which provides notification of news items and new guidance on gov.uk. |
| Shortly after the conclusion of the survey a new quick links menu was added to the website to help with navigation with quick links to the most popular VMD related pages. | |
| A review of MAVIS is being conducted and how information published within MAVIS might be better presented on the website. | |
| The VMD also uses its Twitter Feed to circulate important messages. |
2. Licensing
2.1 Marketing Authorisations
Details of clinically significant variations are published in the Veterinary Record and the Veterinary Times.
You can see lists on GOV.UK of:
2.2 Quarterly reporting against VMD Published Standards for 2018/19 licensing work
This report is published on a monthly basis under Our Statistics on GOV.UK.
For further information contact Natalie Shilling [email protected] and put ‘MAVIS’ in the subject line.
2.3 Top ten imported veterinary medicines - Quarterly report 1 April to 30 June 2018
We provide a list on a quarterly basis of the ten products for which most Special Import and Special Treatment Certificates (SIC and STC) have been issued. This list contains details of the product, the active substance and the number of certificates.
We hope the pharmaceutical industry find this list helpful in considering where there might be a need for a UK authorised product.
| Product | Active Substance | No. of Certificates Issued |
|---|---|---|
| Artuvetrin® Therapy, suspension for subcutaneous injection in dogs | Allergens | 2,473 |
| Aquamid | Polyacrylamide | 426 |
| Spectrum Hyposensitisation Vaccine – Injectable Solution | Allergens | 225 |
| Vet-Goid | Allergens | 220 |
| Ekyflogyl 125mls (Solution of prednisolone 2mg/ml, Lidocaine 0.01g/ml, Dimethyl sulphoxide 0.8ml/ml) | Prednisolone Acetate Lidocaine Hydrochloride Dimethyl Sulphoxide | 99 |
| Oncept (Canine Melanoma Vaccine) | Canine Melanoma DNA | 97 |
| Greer Allergenic Extract Patient Prescription | Allergens | 89 |
| Antepsin 1g Tablets Sucrafalto | Sucralfate | 87 |
| Milteforan 20 mg/ml Solución Oral Para Perros | Miltefosine | 82 |
| Antepsin 20% Oral Suspension | Sucralfate | 75 |
For further information contact Stacey Brown [email protected]
3. Inspections and investigations
Breaches of the Veterinary Medicines Regulations are investigated in accordance with our published Enforcement Strategy.
3.1 Good Manufacturing Practice
Manufacturing sites must comply with Good Manufacturing Practice (GMP) standards. Eudralex Volume 4 provides guidance for the interpretation of the principles and guidelines of good manufacturing practices for medicinal products for human and veterinary use.
3.2 GMP inspection deficiency findings 1 April 2018 to 30 September 2018
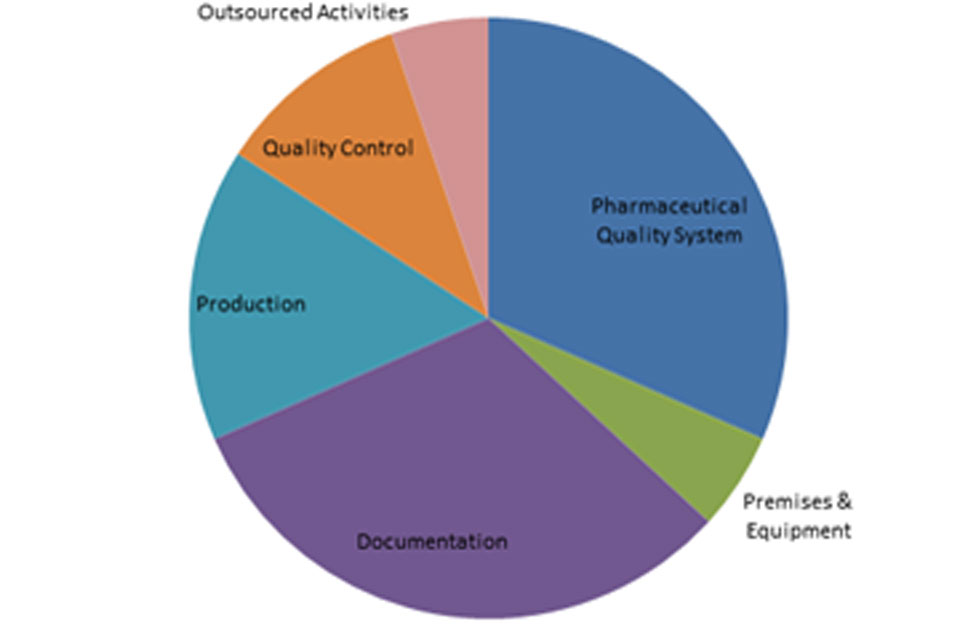
The distribution of inspection deficiencies for each of the nine GMP chapters is shown below. No critical deficiencies were cited in this period:
‘Other’ Deficiencies Apr 18 - Sept 18
GMP Inspection Major Deficiencies April 2018 to September 2018
‘Major’ Deficiencies Apr 18 - Sept 18
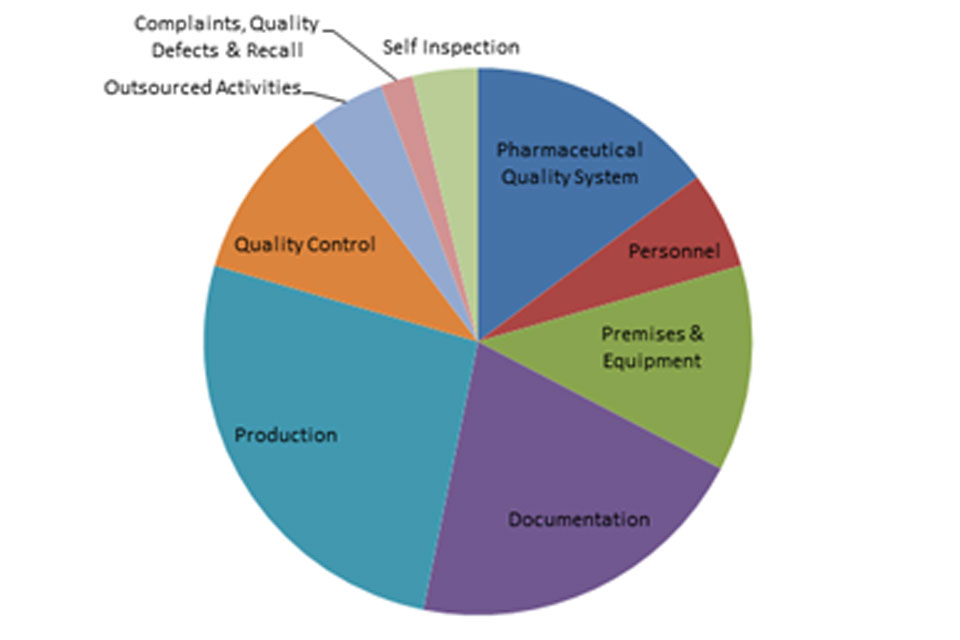
GMP Inspection Other Deficiencies April 2018 to September 2018
4. Pharmacovigilance reports
4.1 Quarterly report
During the period 1 June 2018 to 31 August 2018, the VMD received 1,730 animal suspected adverse event reports. Of the 2,650[footnote 1] products involved in these reports, 2,574 were authorised veterinary medicines which belong to the following distribution categories:
| Amount | Legal Category |
|---|---|
| 2,375 | Prescription Only Medicine Veterinarian (POM V) |
| 117 | Prescription Only Medicine Veterinarian, Pharmacist, SQP (POM VPS) |
| 42 | Non Food Animal Veterinarian, Pharmacist, SQP (NFA VPS) |
| 40 | Authorised Veterinary Medicine General Sales List (AVM GSL) |
The remaining 76 products were:
| Amount | Type |
|---|---|
| 29 | Authorised human medicines |
| 3 | Medicine used in a trial under an Animal Test Certificate |
| 8 | Veterinary products without any medicinal claim |
| 6 | Authorised medicines imported from other countries |
| 3 | Medicines sold under the exemption for small pet animals |
| 9 | Extemporaneous veterinary medicines |
| 18 | Incompletely identified products |
During this period 67 reports of human suspected adverse reactions were received. Of the 76[footnote 1] products involved in these reports, 70 were authorised veterinary medicines which belong to the following distribution categories:
| Amount | Legal Category |
|---|---|
| 55 | Prescription Only Medicine Veterinarian (POM V) |
| 11 | Prescription Only Medicine Veterinarian, Pharmacist, SQP (POM VPS) |
| 3 | Non Food Animal Veterinarian, Pharmacist, SQP (NFA VPS) |
| 1 | Authorised Veterinary Medicine General Sales List (AVM GSL) |
No Environmental Incident reports were received during this period.
For further information contact Roy Savory [email protected]
5. Enforcement
We publish a list of prosecutions and notices involving illegal activity with veterinary medicines in the last year.
You can report information about suspected illegal medicines or breaches of the Veterinary Medicines Regulations to [email protected]. Details of how to report and how we will deal with the reports can be found in the guidance Report illegal animal medicines.
If you have concerns about a non medicinal product such as a product making an unauthorised claim, you can submit them using the Unauthorised Product Complaint Reporting Form.
All information will be treated confidentially.
6. Antimicrobial Resistance
6.1 Defra Antimicrobial Resistance Co-ordination (DARC) group
The DARC group met on 13 September 2018 to discuss the recent trends in antibiotic resistance (AMR) of importance to people and animals. The group received an update on antibiotic consumption data projects under development, as well as recent international collaborations. Topical presentations were given that outlined a One Health approach to AMR surveillance with contributions from the Food Standard Agency, Animal and Plant Health Agency and Public Health England. Summary minutes of the meeting will be published on GOV.UK in due course. Minutes from previous meetings are available.
6.2 Sales Data and Antibiotic Resistance Surveillance Report
The 2017 UK Veterinary Antibiotic Resistance and Sales Surveillance (UK-VARSS) Report has been published. The report collates UK data on antibiotic sales from Marketing Authorisation Holders and antibiotic resistance data from the VMD’s surveillance programmes.
UK-VARSS 2016 and previous reports are also available.
6.3 UK AMR National Action Plan (Strategy)
The next five year AMR national action plan and longer term vision is in its final stages of drafting. The anticipated publication date is early 2019.
6.4 Communications
In September, the Health and Social Care Select Committee launched an inquiry into AMR. In addition to Public Health representatives, the Chief Veterinary Officer, Chief Medical Officer, Lord O’Neill, Gwyn Jones from the Responsible Use of Medicines in Agriculture Alliance (RUMA) and Peter Borriello VMD CEO appeared before the committee to provide evidence on the results delivered by the UK AMR Strategy 2013-2018 and what the key elements for the Government’s next strategy should be. The committee will now write a report in response to the evidence given and suggestions from the report will be incorporated into the Government’s next UK AMR Strategy prior to launch. Transcripts from each session are available.
The VMD are supporting RUMA’s #VaccinesWork campaign. Using social media platforms, facts are being broadcasted to champion vaccination as a preventative infection prevention and disease control method in the animal sector. More information is available on the #VaccinesWork website
The autumn catch-up meeting in preparation for European Antibiotic Awareness Day and World Antibiotic Awareness Week 2018 activities was held on 14 September 2018. One Health colleagues from across government and animal health experts from the private sector gathered to discuss plans and identify areas for collaboration.
For further information contact Zoe Davies [email protected]
7. Veterinary Products Committee
7.1 Meetings of the Veterinary Products Committee (VPC)
The VPC met in September 2018. Summary minutes of the meetings held since October 2014 are available on GOV.UK.
Minutes of meetings held between 2009 and May 2014 are available on the National Archives.
The VPC held its Open meeting on 28 September. A presentation given by one of its members, Professor Jason Weeks is available to view.
For further information contact Sandra Russell [email protected]
8. Residues controls and monitoring
8.1 Results of Statutory Surveillance
Sampling commenced in January 2018 and full details of UK surveillance results, together with information on any action taken, can be found on GOV.UK.
For further information contact Alison Jones [email protected]
9. Staff changes
The following staff changes took place during this quarter:
New staff:
- Mike Mawhinney joined the Inspections and Investigations team as an inspector in Northern Ireland
- Kashif Shah joined the Finance team
Departing staff:
- Javier Pozo, Hannah Reeves, Giles Davis and Bijal Mistry
Movements within the VMD:
- Lisa Bennett and Sarah Jane Gibbons were temporarily promoted within the Pharmaceuticals & Feed Additives team and Ann Baker was temporarily promoted and transferred to the team
- Gavin Hall was temporarily promoted and transferred to the EU Exit team and Guy Glover temporarily transferred to the team
- David Lewsey and Sandra Russell were temporarily promoted within the Business Support Division
- Yolanda Ayres was temporarily promoted and Alison Reynolds transferred to the Inspections and Investigations team. Julia Pearce was promoted within the team.
- Myles Munro was promoted and transferred to the Legislation team and Anna Burrows was temporarily promoted within the team
- Marissa Ashfield temporarily transferred to the Enforcement team
- Alison Jones was promoted and transferred to the Residues team
10. Organogram as at 30 September 2018
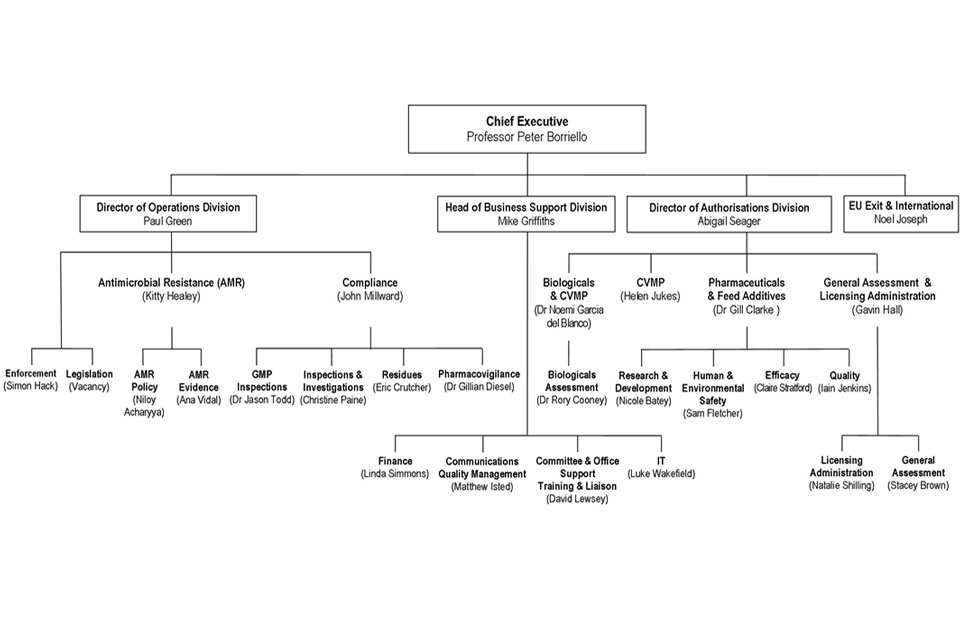
VMD Organogram as at September 2018
