EMHP wastewater monitoring of SARS-CoV-2 in England: 1 June to 1 November 2021
Published 25 November 2021
Data showing the concentration of SARS-CoV-2 RNA detected in wastewater by the Environmental Monitoring for Health Protection (EMHP) wastewater monitoring programme.
Background on the EMHP wastewater monitoring programme
People infected with coronavirus (COVID-19) shed the virus during daily activities such as going to the toilet and blowing noses. The virus enters the sewer system through sinks, drains and toilets. Fragments of the SARS-CoV-2 virus (the virus that causes COVID-19) can be detected in samples of wastewater (untreated sewage).
The EMHP programme, led by the UK Health Security Agency (UKHSA), tests sewage for fragments of SARS-CoV-2 RNA. Untreated influent samples are taken from approximately 270 sewage treatment works (STWs) in England, 4 times a week (see figures 1 to 9). EMHP also samples from sewer network sites (manholes). Data from sewer network sites is not included in this publication as these sites generally fall within sewage treatment work (STW) catchments. Further detail about EMHP wastewater coverage can be found in the publication Wastewater testing coverage data for the Environmental Monitoring for Health Protection (EMHP) programme.
The programme helps identify where the virus is circulating in England, detecting spikes in prevalence and presence of variants. Findings are reported to national decision makers and local stakeholders on a regular basis, helping to inform national strategy and localised action. Wastewater analysis has the benefit of detecting the virus regardless of whether people have symptoms or whether they have a test. Wastewater monitoring complements other testing programmes and public health actions to help protect against the threat of new variants.
As the threat of variants has emerged, the programme plays a key role in the detection of mutations of the virus, variants of concern (VOCs) and variants under investigation (VUIs). This is achieved through genomic sequencing of wastewater samples, to provide an indication of where VOCs and VUIs may be present across England. Insights from wastewater monitoring are shared with local and national decision makers, to help inform the action that they can take to stop further transmission.
The programme is led by UKHSA and run in partnership with the Department for Environment, Food and Rural Affairs (Defra), the Environment Agency (EA), the Centre for Environment, Fisheries and Aquaculture Science (Cefas), academia, and water companies. It provides coverage of approximately 40 million people across England. The EMHP team coordinates with programmes in the devolved administrations to provide UK-wide wastewater monitoring.
EMHP advises caution when directly comparing data from STWs and concentration of SARS-CoV-2 RNA in wastewater with clinical testing data. Interpretation should be made with careful consideration of the points in the Uncertainty, data quality, and revisions section.
EMHP sampling sites
EMHP samples at sewer network sites (manholes in the street which generally serve a local area) and STWs (which generally serve wider areas such as a city or town). Associated with every STWs and sewer network site is a catchment, the geographical area from which wastewater flows into the sampling location. This publication reports data from STWs. Data from sewer networks is not included because sewer network sites generally fall within the catchment of an STW. In addition, EMHP changes sampling strategy and locations of sewer network sites to meet epidemiological priorities (for example to support local public health responses).
Figures 1 to 9 show the location of STWs EMHP sample in each region.
Figure 1. Map showing the location of Sewage Treatment Works sampled in the East Midlands.
Location of STWs sampled in the East Midlands:
- Alfreton
- Anwick
- Boston
- Bourne
- Brackley
- Chesterfield
- Corby
- Daventry
- Derby
- Grantham
- Ingoldmells
- Leicester
- Lincoln
- Loughborough
- Louth
- Mablethorpe
- Market Harborough
- Mansfield
- Melton Mowbray
- Northampton
- Nottingham
- Oakham
- Retford
- Spalding
- Stamford
- Wellingborough
- Whaley Bridge
- Wigston
- Worksop
Figure 2. Map showing the location of Sewage Treatment Works sampled in the East of England.
Location of STWs sampled in the East of England:
- Basildon
- Beccles
- Bedford
- Braintree
- Breckland
- Bury St Edmunds
- Cambridge
- Chalton
- Chelmsford
- Colchester
- Cromer
- Diss
- Downham Market
- Felixstowe
- Great Yarmouth
- Harlow
- Haverhill
- Hunstanton
- Huntingdon
- Ipswich
- Jaywick
- Kings Lynn
- Letchworth
- Lowestoft
- Luton
- March
- Needham Market
- Newmarket
- Norwich
- Peterborough
- Radlett
- Reepham
- Royston
- Saffron Walden
- Shefford
- Soham
- St Albans
- Stalham
- Stowmarket
- Sudbury
- Southend-on-Sea
- Thetford
- Tilbury
- Wells-next-the-Sea
- Wisbech
- Witham
Figure 3. Map showing the location of Sewage Treatment Works sampled in London.
Location of STWs sampled in London:
- Beckton
- Beddington
- Crossness
- Deepham
- Hogsmill Valley
- Long Reach
- Mogden
- Riverside

Figure 4. Map showing the location of Sewage Treatment Works sampled in the North East.
Location of STWs sampled in the North East:
- Billingham
- Bishop Auckland
- Consett
- Cramlington
- Darlington
- Hartlepool
- Horden
- Middlesbrough
- Newcastle
- Newton Aycliffe
- Sunderland
- Washington
Figure 5. Map showing the location of Sewage Treatment Works sampled in the North West.
Location of STWs sampled in the North West:
- Barnoldswick
- Barrow-in-Furness
- Blackburn
- Bolton
- Burnley
- Bury
- Carlisle
- Clitheroe
- Congleton
- Crewe
- Ellesmere Port
- Fleetwood
- Hyde
- Hyndburn
- Kendal
- Lancaster
- Leigh
- Liverpool (Sandon)
- Macclesfield
- Maghull
- Northwich
- Penrith
- Preston
- Rochdale
- Rossendale
- Skelmersdale
- Stockport
- Walton-Le-Dale
- Warrington
- Wigan
- Wirral
- Workington

Figure 6. Map showing the location of Sewage Treatment Works sampled in the South East.
Location of STWs sampled in the South East:
- Andover
- Alton
- Ashford
- Aylesbury
- Banbury
- Basingstoke
- Bexhill
- Bicester
- Bordon
- Buckingham
- Burgess Hill
- Camberley
- Canterbury
- Chatham
- Chesham
- Chichester
- Crawley
- Didcot
- Dover and Folkestone
- Eastleigh
- Fareham and Gosport
- Guildford
- Hailsham
- Hythe
- Isle of Wight
- Lewes
- Littlehampton and Bognor
- Lymington and New Milton
- Maidstone and Aylesford
- Milton Keynes
- Newbury
- New Forest
- Oxford
- Portsmouth and Havant
- Ramsgate, Sandwich and Deal
- Reading
- Reigate
- Scaynes Hill
- Sittingbourne
- Slough
- Southampton
- Tonbridge
- Tunbridge Wells
- Witney
- Woking
- Worthing
- Wycombe

Figure 7. Map showing the location of Sewage Treatment Works sampled in the South West.
Location of STWs sampled in the South West:
- Barnstaple
- Bath
- Bideford
- Blandford Forum
- Bodmin Sc Well
- Bournemouth (Central)
- Bridport
- Bristol
- Camborne
- Chard
- Cheltenham
- Chippenham
- Cirencester
- Clevedon and Nailsea
- Ernesettle and Saltash
- Exmouth
- Falmouth
- Gloucester
- Helston
- Liskeard
- Lydney
- Menagwins
- Minehead
- Newquay
- Newton Abbot
- Par
- Plymouth (Camels Head)
- Plymouth
- Plympton
- Salisbury
- Shaftesbury
- Shepton Mallet
- Sidmouth
- St Ives and Penzance
- Stroud
- Swanage
- Swindon
- Taunton
- Tavistock
- Tiverton
- Torquay
- Trowbridge
- Wellington
- Weston-super-Mare
- Weymouth
- Yeovil

Figure 8. Map showing the location of Sewage Treatment Works sampled in the West Midlands.
Location of STWs sampled in the West Midlands:
- Barston
- Brancote
- Checkley
- Birmingham (Coleshill)
- Birmingham (Minworth)
- Burton on Trent
- Coventry
- Evesham
- Kidderminster
- Leek
- Ludlow
- Malvern
- Market Drayton
- Nuneaton
- Oswestry
- Rugby
- Spernal
- Stoke-on-Trent
- Stourbridge and Halesowen
- Telford
- Telford South
- Walsall
- Warwick
- Wolverhampton
- Worcester
Figure 9. Map showing the location of Sewage Treatment Works sampled in Yorkshire and the Humber.
Location of STWs sampled in Yorkshire and the Humber:
- Barnsley
- Barton-upon-Humber
- Beverley
- Bradford
- Bridlington
- Colburn
- Dewsbury
- Doncaster (Thorne)
- Doncaster (Sandall)
- Driffield
- Grimsby
- Harrogate
- Huddersfield
- Hull
- Keighley
- Leeds
- Malton
- Northallerton
- Pontefract
- Scarborough
- Scunthorpe
- Sheffield (Blackburn Meadows)
- Sheffield (Woodhouse Mill)
- Wakefield
- York
Concentration of SARS-CoV-2 RNA in wastewater samples
EMHP wastewater concentration data from 1 June to 1 November 2021 is available in the accompanying spreadsheet. The spreadsheet includes data from previous months’ publications, along with the latest October data. The most recent 6 weeks of data are visualised in figures 10 to 12; England concentration map. Note that STWs are omitted from figures 10 to 12 if no samples were taken during the week.
From September 2021 concentrations of SARS-CoV-2 RNA in wastewater measured by EMHP have appeared to be less consistently aligned with reported clinical data. EMHP is continuing to investigate the relationship between COVID-19 cases and SARS-CoV-2 concentrations in wastewater, and how this may be fluctuating over time as the pandemic develops.
In the week beginning 21 September, 151 of 268 STWs (55%) had higher average concentration compared to the previous week. In the week beginning 28 September, 197 of 266 STWs (74%) had lower average concentration compared to the previous week. In the week beginning 5 October, 152 of 266 STWs (56%) had higher average concentration compared to the previous week. In the week beginning 12 October, 167 of 267 STWs (63%) showed a decrease in weekly average concentration. In the week beginning 19 October, 165 of 269 STWs (61%) showed an increase in average concentration compared to the previous week. In the last week of October, 147 of 271 STWs (54%) had lower average concentration compared to the previous week. Furthermore, any interpretation should be made with careful consideration of the points in the Uncertainty, data quality and revisions section below.

Figure 10. England concentration map: map showing the location of STWs sampled. Colour indicates the weekly-average SARS-CoV-2 RNA concentration (gene copies/litre) in the last 2 weeks. See regional maps for the names of each STWs.

Figure 11. England concentration map: map showing the location of STWs sampled. Colour indicates the weekly-average SARS-CoV-2 RNA concentration (gene copies/litre) in the mid 2 weeks. See regional maps for the names of each STWs.

Figure 12. England concentration map: map showing the location of STWs sampled. Colour indicates the weekly-average SARS-CoV-2 RNA concentration (gene copies/litre) in the first 2 weeks. See regional maps for the names of each STWs.
Regional average concentration of SARS-CoV-2 RNA in wastewater
The concentration of SARS-CoV-2 RNA in wastewater increased in every region from late June to late August. Over the month of September, the concentration of SARS-CoV-2 RNA in wastewater decreased slightly, however this is not deemed to be outside of error bounds. Over the month of October, a decreasing trend continued until mid-October where concentration of SARS-CoV-2 RNA in wastewater increased across all regions. However, there is high variability in concentration between individual STWs. Interpretation should be made with careful consideration of the points in the Uncertainty, data quality, and revisions section.
Figure 13 to 21 show the 7-day rolling average SARS-CoV-2 concentration detected in wastewater for each region in England, together with the national average of SARS-CoV-2 concentration detected in wastewater and individual site data. Samples where no SARS-CoV-2 was detected are assigned the value 160 (gc/l) which is the Theoretical Limit of Detection. Data is weighted by catchment population to account for varying catchment sizes. For further information, see methodology section below.
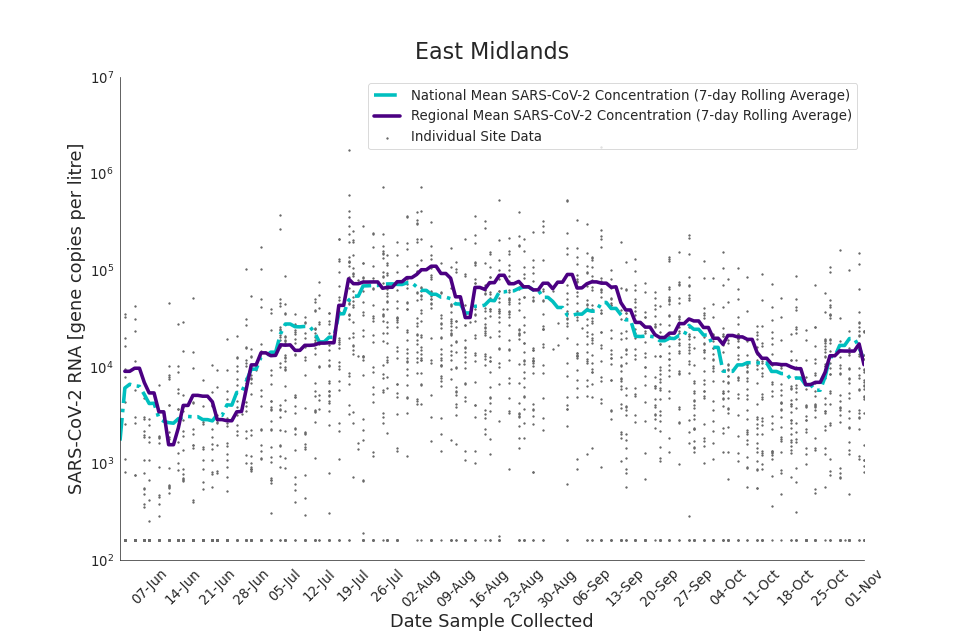
Figure 13. East Midlands 7-day rolling average SARS-CoV-2 RNA concentration
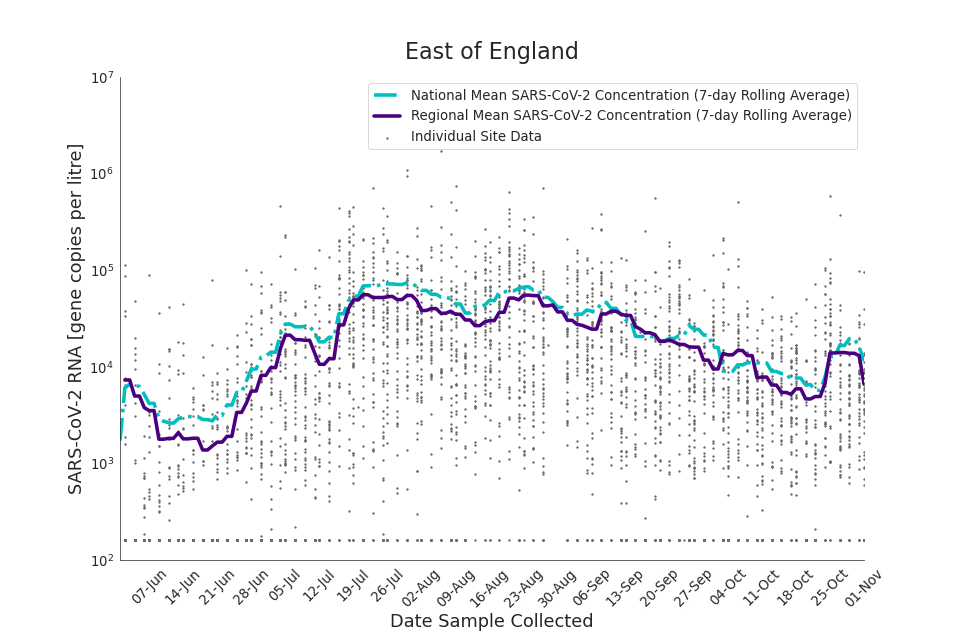
Figure 14. East of England 7-day rolling average SARS-CoV-2 RNA concentration
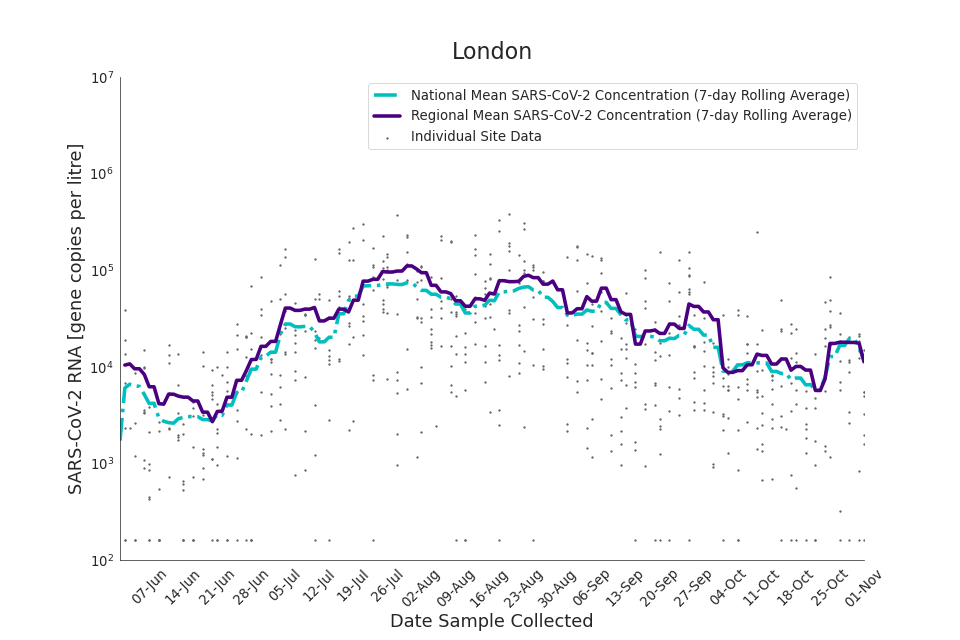
Figure 15. London 7-day rolling average SARS-CoV-2 RNA concentration
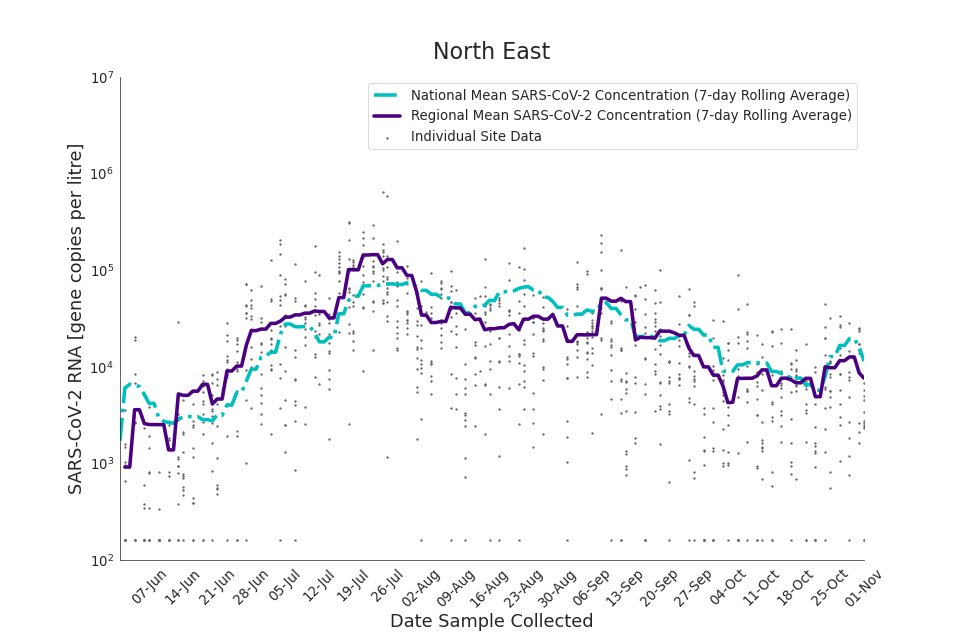
Figure 16. North East 7-day rolling average SARS-CoV-2 RNA concentration
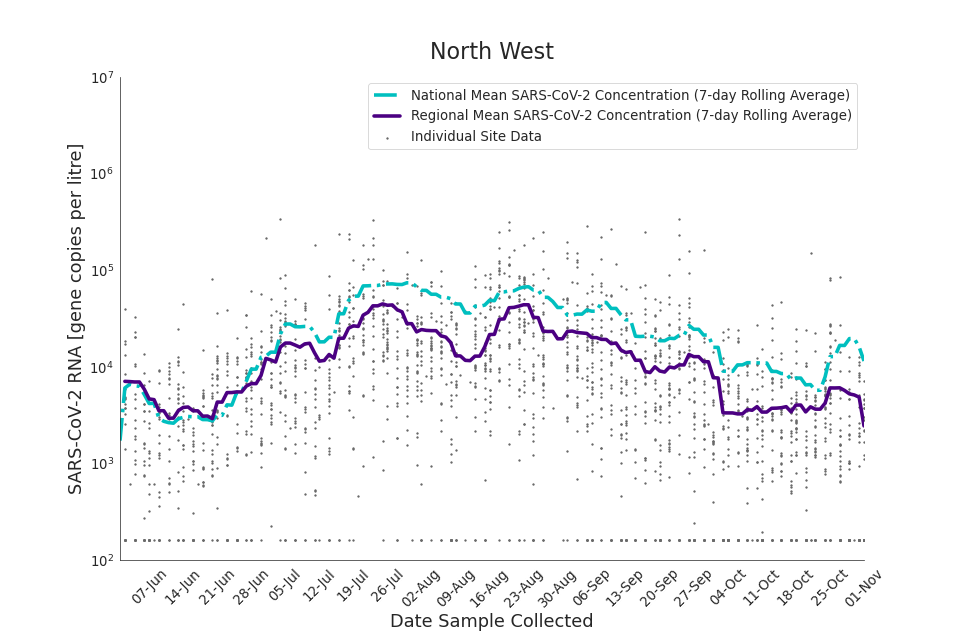
Figure 17. North West 7-day rolling average SARS-CoV-2 RNA concentration
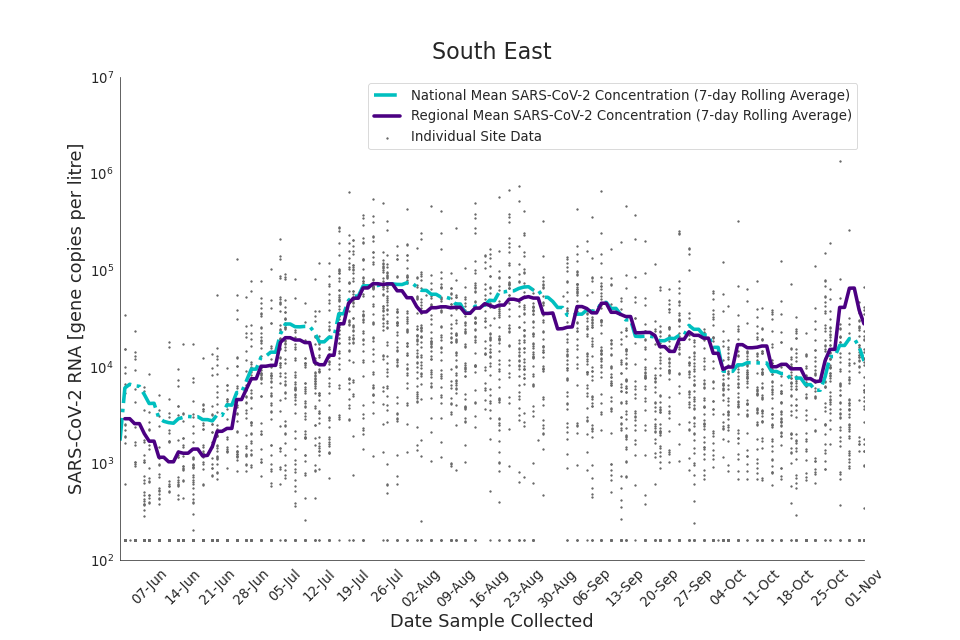
Figure 18. South East 7-day rolling average SARS-CoV-2 RNA concentration
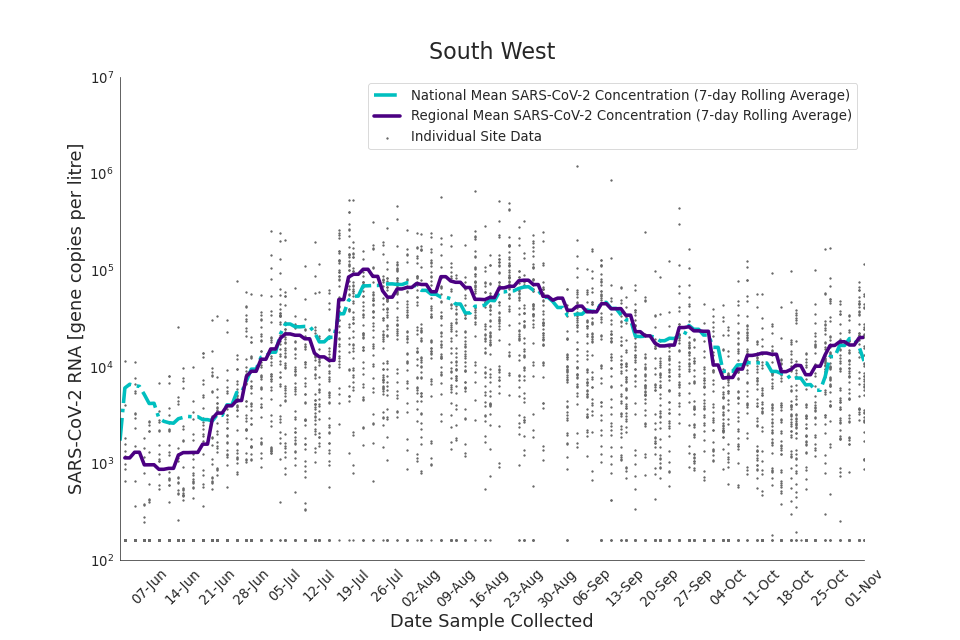
Figure 19. South West 7-day rolling average SARS-CoV-2 RNA concentration

Figure 20. West Midlands 7-day rolling average SARS-CoV-2 RNA concentration
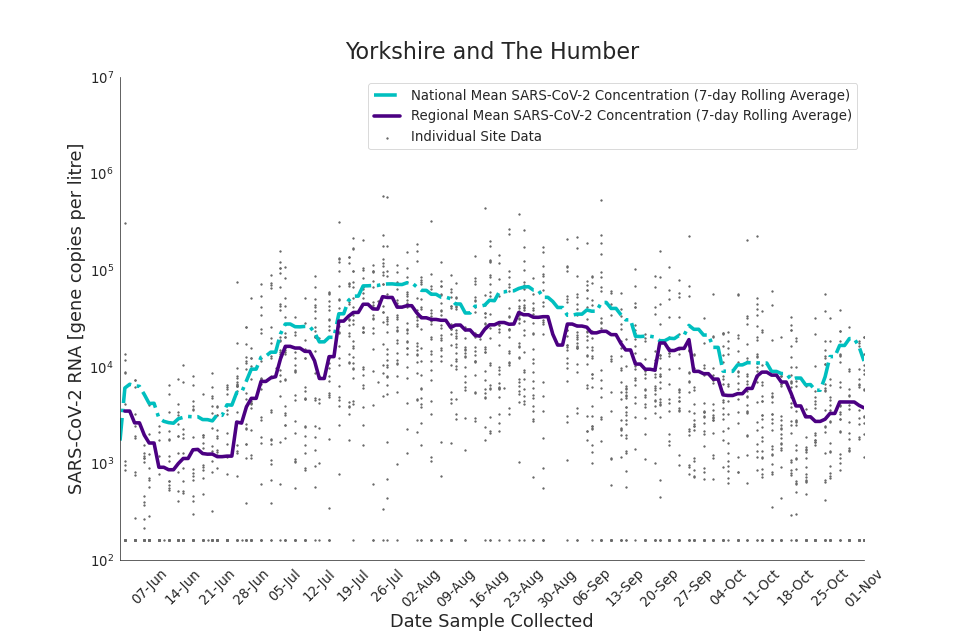
Figure 21. Yorkshire and the Humber 7-day rolling average SARS-CoV-2 RNA concentration
Methodology
About this data
EMHP samples wastewater from STWs throughout England. Samples are analysed for the concentration of SARS-CoV-2 by quantifying the number of copies of the nucleocapsid gene (N1). Samples are transported to Environment Agency laboratory for quantification of the N1 gene using a process called Reverse Transcriptase Polymerase Chain Reaction. The data reported here is the number of SARS-CoV-2 N1 gene copies per litre of wastewater (shortened to gene copies/litre throughout this document). Generally, the more people with COVID-19 in the community the more viral RNA will be shed into wastewater. Therefore, the concentration in wastewater is indicative of the prevalence of COVID-19 in the community.
Samples are analysed for the concentration of SARS-CoV-2 RNA and concentration is adjusted for flow (see Uncertainty, data quality, and revisions). Typically, 4 samples per week are analysed from each STW. However, occasionally fewer samples will be collected and/or analysed. This can be due to several reasons such as EMHP altering sampling strategy because of testing capacity constraints, changing epidemiological priorities and current local response activities.
Weekly Concentration Table and Daily Concentration Table show the concentration of SARS-CoV-2 RNA in wastewater samples (gene copies/litre). Note that there is sensitivity associated with the analysis of wastewater; the Limit of Detection (LOD) is the minimum concentration of SARS-CoV-2 RNA that can be reliably detected. Data tables contain the values ‘tLOD’ where analysis of the sample did not detect presence of SARS-CoV-2 RNA. For the purposes of averaging data to produce weekly averages in the Weekly Concentration Table and Figures 10 to 12, EMHP assigns a concentration of 160 gene copies/litre to all ‘tLOD’ samples. This concentration is the Theoretical Limit of Detection and is calculated by the Environment Agency. Note that there is uncertainty in this calculation and continuing work to refine it, please see the Uncertainty, data quality, and revisions section for further information.
To calculate region averages, data from each STW was weighted by catchment population. To estimate the population of STWs, the catchment areas of STWs are matched to lower layer super output areas (LSOA)s. Catchment areas were provided by the 9 water companies serving England. See the EMHP publication Wastewater testing coverage data for the EMHP programme for a detailed description of this method. Regional and national averages were calculated by taking the 7-day rolling arithmetic mean. Figures are displayed on a logarithmic scale for ease of interpretation.
We are exploring the feasibility of adding more data in future releases, such as detection of variants.
Data sources
Wastewater samples are collected by water companies and transported to an Environment Agency Laboratory. Concentration data is from Environment Agency analysis of wastewater samples.
Figures in this publication show the location of STWs. These locations were obtained from the European Commission urban wastewater website. Note that names differ between those used in this document and the European Commission urban wastewater website, sites can be linked using the ‘STW Site Code’ in the attached data tables.
The figures and analysis presented will evolve over time to ensure the most relevant information is included and the needs of stakeholders are met. These figures will initially be published monthly, from June 2021, and we will continuously evaluate the frequency and date range based on need and public interest.
How these figures can be used
These figures and data can be used to view the concentration of SARS-CoV-2 RNA in wastewater, which is indicative of prevalence in the community. Due to factors relating to data uncertainty, EMHP recommends careful interpretation of this data alongside other data sources such as Weekly Statistics for NHS Test and Trace (England).
Uncertainty, data quality, and revisions
The figures here are compiled by professional analysts and have been quality assured. However, the analytical pipeline to produce this data should still be considered experimental; it is subject to an ongoing quality assurance process. Any revisions to past publications will be in line with DHSC’s revision policy and highlighted in future publications accordingly. The sites from which EMHP sample can change on a regular basis depending on testing capacity, epidemiological priorities, and current local response activities. This can result in changes to specific STWs covered. This means the STW included in subsequent publications may change.
This publication shows STWs as single points. Every STW has an associated geographical catchment area (area from which wastewater flows into the sampling location). These catchments are not yet presented in these statistics publications. There is uncertainty surrounding catchment geography, as catchments vary in size and population covered, and in some cases an STW may not be located within the catchment it serves (for example, if wastewater is pumped to the STW). Therefore, the location of STW should be used as a general guide only.
Wastewater is an inherently variable material and can change greatly within and between days (foodstuffs being consumed; when washing machines are switched on and many other factors), the physical infrastructure and the type and levels of industrial wastes. It is generally more useful to consider trends over medium- and long-term periods of time, rather than between individual days.
There are several factors that can impact the quality of the signal recorded from wastewater and introduce uncertainty.
- The concentration of SARS-CoV-2 RNA in wastewater may be impacted by weather, for example heavy rainfall wastewater may cause dilution. EMHP mitigates this by adjusting concentration to consider flow.
- Whether samples are ‘composite’ or ‘grab’. Composite samples are where an autosampler (a machine which gathers wastewater at regular intervals over a set time period) gathers wastewater throughout the day. Grab samples are where a single sample is taken at one point in the day and are subject to greater variability; they are influenced by flow characteristics, time of day, and are more susceptible to outliers in the sewage. EMHP aims to mitigate this by sampling during times of peak load and ensuring the sample is collected mid-stream rather than close to the edges or bottom of the pipe.
- Calculating the Limit of Detection (LOD; the minimum concentration of SARS-CoV-2 RNA that can be reliably detected in a wastewater sample) can be achieved by several methods. In this data we have used the Theoretical LOD as calculated by the Environment Agency. The Theoretical LOD uses several assumptions. Laboratory data is required to inform the more realistic Practical LOD. The Practical LOD for this analysis is not yet reported here. It is likely that the LOD used in producing these statistics will change in future publications. EMHP will clearly state when this occurs.
- Although the exact value of the LOD is uncertain, it is unlikely to have a noticeable impact on the interpretation of this data. This is because the difference between Theoretical and Practical LOD is relatively small, with most impact at low levels of the target being measured.
- As explained in the ‘About this data’ section, typically 4 wastewater samples are taken at each STW per week. The average of all samples within the week is presented in the Weekly Concentration Table. Uncertainty occurs where fewer samples are taken. For example, if only one sample is taken during a week, that one sample defines the week average. This means weeks with fewer samples are more susceptible to variability and outliers. Sample Count Table shows how many samples were taken at an STW for each week in this release.
For the above reasons EMHP advises caution when directly comparing data from STWs and concentrations of SARS-CoV-2 RNA in wastewater with clinical testing data.
Methodology and limitations will differ across data sources and this should be taken into account.
If you would like to give user feedback, please email [email protected]
