Quarterly vaccination coverage statistics for children aged up to 5 years in the UK (COVER programme): July to September 2022
Updated 26 September 2023
This report of the cover of vaccination evaluated rapidly (COVER) programme presents quarterly coverage data for children in the UK who reached their first, second, or fifth birthday during the evaluation quarter (July to September 2022).
This report is the first in the series to be published in HTML format. The full coverage data – broken down by country, NHS England local team (configuration as at 1 April 2018) and NHS England region – was previously contained in appendices to the main report. It is now contained in the separate data file accompanying this report along with data by upper tier local authority and UKHSA region and can be found on the landing page for this report. For a PDF version of this report please email [email protected]
1. Main points
All comparisons are with the previous quarter:
- 12-month UK coverage for the ‘6-in-1’ vaccine remained at 92.4%, rotavirus decreased by 0.3% to 89.4%, MenB2 increased by 0.2% to 92.3% and PCV dose 1 increased by 0.1% at 94.3%
- in England, ‘6-in-1’ coverage increased by 0.1% to 92.1%, MenB increased by 0.1% to 91.9%, rotavirus decreased by 0.3% to 89.0% and PCV1 coverage decreased by 0.1% to 94.1%
- at the country-level, in Scotland at least 95% coverage was achieved for all antigens (except rotavirus) at 12 months, in Wales at least 93% coverage, and in Northern Ireland coverage was at least 91%
- in the UK, 24-month coverage of the ‘6-in-1’ vaccine decreased 0.1% to 93.3%, MMR1 decreased by 0.1% at 90.0%, Hib/MenC decreased 0.1% to 90.2%, coverage for PCV remained at 89.9% and MenB remained at 89.1%
- at 24 months, coverage in England for the ‘6-in-1’ vaccine remained at 92.9%, decreased by 0.1% to 89.5% for Hib/MenC, increased by 0.1% to 88.6% for MenB, increased by 0.1% to 89.4% for the PCV booster and remained at 89.7% for MMR1
- at the country level, coverage in Scotland exceeded 93% for all the vaccines offered from the first birthday, in Wales it exceeded 92% and in Northern Ireland and in England, coverage exceeded 88%
- at 5 years, UK coverage for the pre-school booster (DTaP/IPV) increased by 0.3% to 84.4%, MMR2 increased by 0.2% to 85.5% and the Hib/MenC booster decreased by 0.1% to 91.7%
- this is the second report for which BCG coverage has been collected for all eligible children in England measured at 3 months, coverage in England was 67.5% compared with 63.1% last quarter
Coverage evaluated at the first, second and fifth birthdays, by country, NHS England local teams (configuration as of 1 April 2018), NHS England regions, upper tier local authorities and UKHSA regions are described in the accompanying data file.
2. Scope
Children who reached their first birthday in this quarter would have been scheduled to complete the primary vaccination courses. This comprises a third dose of the DTaP/IPV/Hib/HepB3 (‘6-in-1’) vaccination which protects against diphtheria, tetanus, pertussis (whooping cough), polio, haemophilus influenzae type b (Hib) and hepatitis B and a second dose of MenB vaccine which protects against Meningococcal group B disease at the age of 16 weeks, between November 2021 and January 2022. They would have also been scheduled to receive a single dose of PCV (protecting against pneumococcal disease) and 2 doses of rotavirus vaccine by age 12 weeks, between October 2021 and December 2021.
With the exception of the rotavirus vaccine, which is only offered up to 6 months of age, all other vaccines are available to children in the current cohort at any time and would have been captured in this report if given by their first birthday. Children born to hepatitis B surface antigen (HBsAg) positive mothers who reached their first birthday in this quarter should also have received monovalent hepatitis B vaccine at birth and at 4 weeks of age.
Children who reached their second birthday would have been scheduled to complete their primary course (third dose) of the ‘6-in-1’ vaccination between November 2020 and January 2021. They also would have been scheduled their first measles, mumps, and rubella (MMR) vaccination, a Hib/MenC booster (protecting against Hib and Meningococcal group C disease), MenB booster and PCV booster at age one year between July and September 2021. Children born to HBsAg positive mothers, who reached their second birthday in this quarter (born July to September 2020), were scheduled to receive a third dose of the monovalent hepatitis B vaccine at one year of age.
Children who reached their fifth birthday would have been scheduled to complete their primary course (third dose) of the ‘6-in-1’ vaccination between November 2017 and January 2018, receive their first MMR and the Hib/MenC booster between July and September 2018, their pre-school diphtheria, tetanus, acellular pertussis and polio (DTaP/IPV) booster, and second-dose MMR from November 2020 to January 2021.
Children born in areas where the TB (tuberculosis) incidence is greater than or equal to 40 per 100,000 or who were born to parents or grandparents from TB endemic areas were eligible for a BCG vaccination at 28 days. Coverage is measured at 3 months of age for this selective immunisation. The full routine immunisation schedule sets out the schedule for all childhood immunisations.
3. Results
This publication is released on a quarterly basis and aligns with financial quarters. The analysis follows this pattern; any discussion of quarters aligns with the financial year whereby quarter 1 starts in April.
3.1 Coverage at 12 months
Compared with the previous quarter, UK coverage for the ‘6-in-1’ (DTaP/IPV/Hib/HepB3) vaccine remained at 92.4%, rotavirus decreased by 0.3% to 89.4% while MenB2 increased by 0.2% to 92.3% (1). This is the seventh quarterly cohort to be routinely offered one PCV dose in the first year of life at 12 weeks and the UK coverage increased by 0.1% to 94.3%.
In England, 12-month coverage of the ‘6-in-1’ increased by 0.1% to 92.1%, rotavirus decreased by 0.3% to 89.0% and MenB increased by 0.1% to 91.9%. PCV1 coverage increased by 0.1% to 94.1%.
With the exception of the rotavirus vaccine, in Scotland at least 95% coverage was achieved for all antigens at 12 months, at least 94% coverage in Wales, and in Northern Ireland coverage was at least 91%.
Full coverage data by country, NHS England local team (configuration as at 1 April 2018), NHS England region, upper tier local authority and UKHSA region is contained in the separate data file that can be found on the landing page for this report.
In England, coverage for the ‘6-in-1’ vaccine peaked at 94.7% in quarter 2 (July to September) of 2012 to 2013, at 92.1% this quarter was 2.6% lower than the peak. Rotavirus coverage this quarter was 1.9% lower than the peak of 90.9% in quarter 1 (April to June) of 2020 to 2021 while MenB coverage was 1.1% lower than the peak of 93.0% in quarter 3 (October to December) of 2017 to 2018.
Figure 1. Completed primary immunisations in England at 12 months between financial quarter 2 (July to September) 2012 to 2013 and quarter 2 2022 to 2023
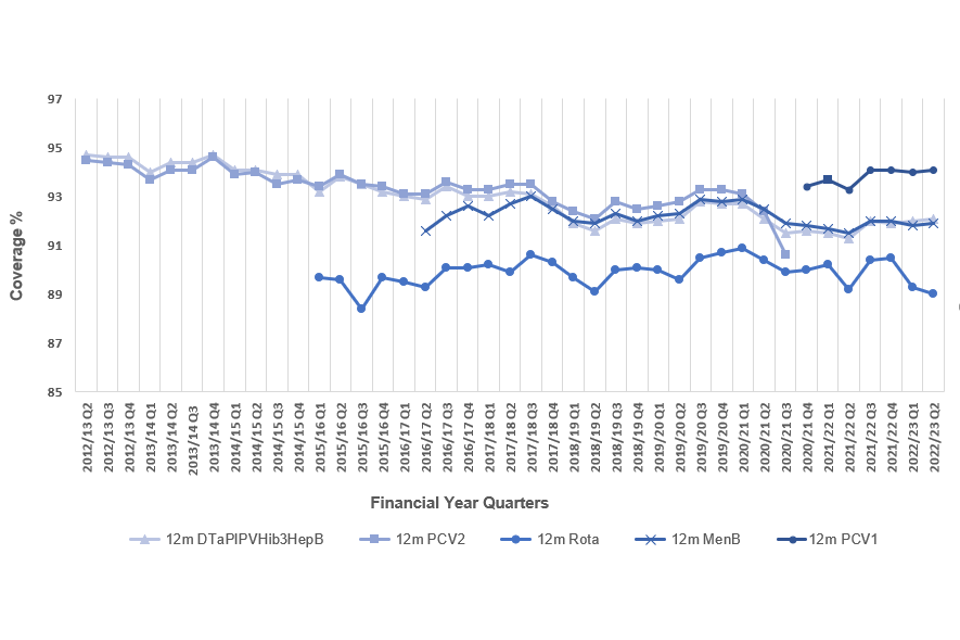
Note: From quarter 4 (January to March) 2020 to 2021 we started to report PCV1 rather than PCV2 to reflect the change in the PCV schedule.
3.2 Coverage at 24 months
In the UK, coverage of the ‘6-in-1’ vaccine decreased 0.1% to 93.3%, MMR1 decreased by 0.1% to 90.1%, and Hib/MenC decreased 0.1% to 90.0%. Coverage for PCV remained at 89.9% and MenB at 89.1%.
Compared with the previous quarter, coverage in England for the ‘6-in-1’ vaccine remained at 92.9%, decreased by 0.1% to 89.5% for Hib/MenC and increased by 0.1% to 88.6% for MenB. Coverage for the PCV booster increased by 0.1% to 89.4%. Coverage for MMR1 remained at 89.7%.
At the country level, coverage in Scotland exceeded 93% for all the vaccines offered from the first birthday, in Wales it exceeded 92% and in Northern Ireland and in England, coverage exceeded 88%.
Full coverage data by country, NHS England local team (configuration as at 1 April 2018), NHS England region, upper tier local authority and UKHSA region is contained in the separate data file that can be found on the landing page for this report.
Figure 2. Completed primary immunisations in England at 24 months between financial quarter 2 (July to September) 2012 to 2013 and quarter 2 2022 to 2023
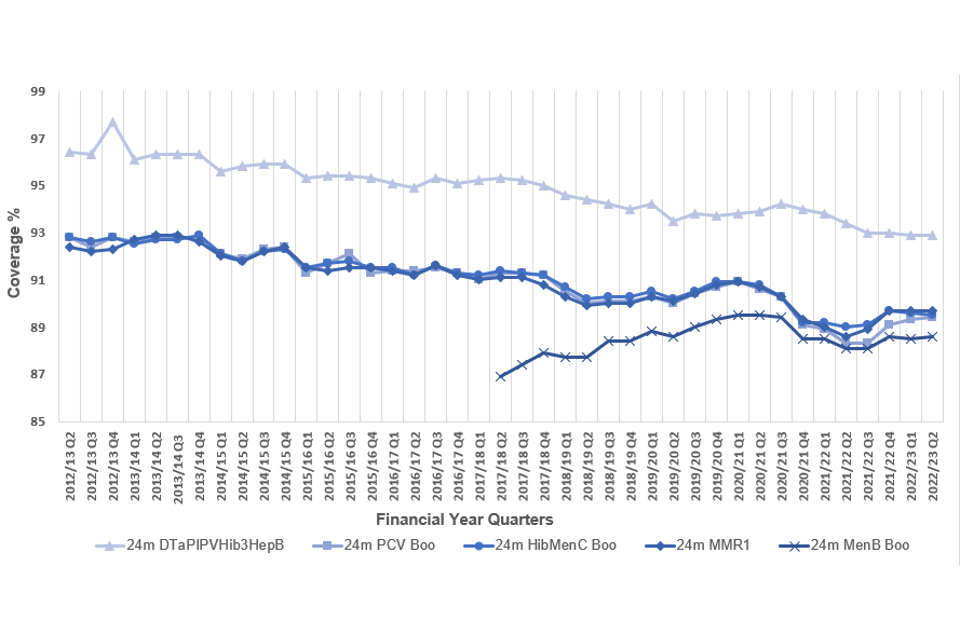
In England, coverage for the ‘6-in-1’ vaccine peaked at 97.7% in quarter 4 (January to March) of 2012 to 2013 and this quarter was 4.8% lower than the peak at 92.9%. PCV booster coverage was 3.4% lower this quarter than the peak of 92.8% in quarter 4 of 2012 to 2013. Hib/MenC coverage was 3.4% lower than the peak of 92.9% in quarter 4 of 2013 to 2014, while MMR1 coverage was 3.2% lower than the peak of 92.9% in quarter 3 (October to December) of 2013 to 2014. MenB coverage was 0.9% lower than the peak of 89.5% in quarter 2 (July to September) of 2020 to 2021.
3.3 Coverage at 5 years
Both Scotland and Wales achieved the 95% World Health Organisation (WHO) target for DTaP/IPV/Hib3 at 5 years. However, in England coverage this quarter decreased by 0.5% compared with the previous quarter and was at 93.5% while coverage for the UK as a whole decreased by 0.5% to 93.9%. This target was also achieved for MMR1 in Scotland and Wales. In England, MMR1 coverage was 92.9%. Coverage at 5 years for these vaccines primarily reflects vaccinations delivered 4 years ago.
MMR2 and the preschool booster are given from age 3 years and 4 months and reflect vaccinations that should have been delivered between November 2020 and January 2021.
Compared with the previous quarter, UK coverage for the pre-school booster (DTaP/IPV) increased by 0.3% to 84.4%, MMR2 increased by 0.2% to 85.5% and the Hib/MenC booster decreased by 0.1% to 91.7%.
In England, coverage for MMR2 increased by 0.3% to 84.7% and the pre-school booster increased by 0.4% to 83.4%. Pre-school booster and MMR2 coverage exceeded 90% in Scotland and Wales.
Full coverage data by country, NHS England local team (configuration as at 1 April 2018), NHS England region, upper tier local authority and UKHSA region is contained in the separate data file that can be found on the landing page for this report.
In England, coverage of DTaP/IPV/Hib3 was 2.5% lower this quarter than the peak of 96.0% in quarter 2 (July to September) of 2012 to 2013. MMR1 was down by 2.7% from a peak of 95.6% in quarter 1 (April to June) of 2017 to 2018. MMR2 was down by 3.9% when compared with the 88.6% seen in quarter 1 of 2014 to 2015. The pre-school booster was 5.8% lower than at its peak of 89.2% in quarter 2 of 2012 to 2013 and Hib/MenC was 1.9% lower than the peak of 93.1% in quarter 2 of 2017 to 2018.
Figure 3. Completed primary immunisations in England at 5 years between financial quarter 2 (July to September) 2012 to 2013 and quarter 2 2022 to 2023
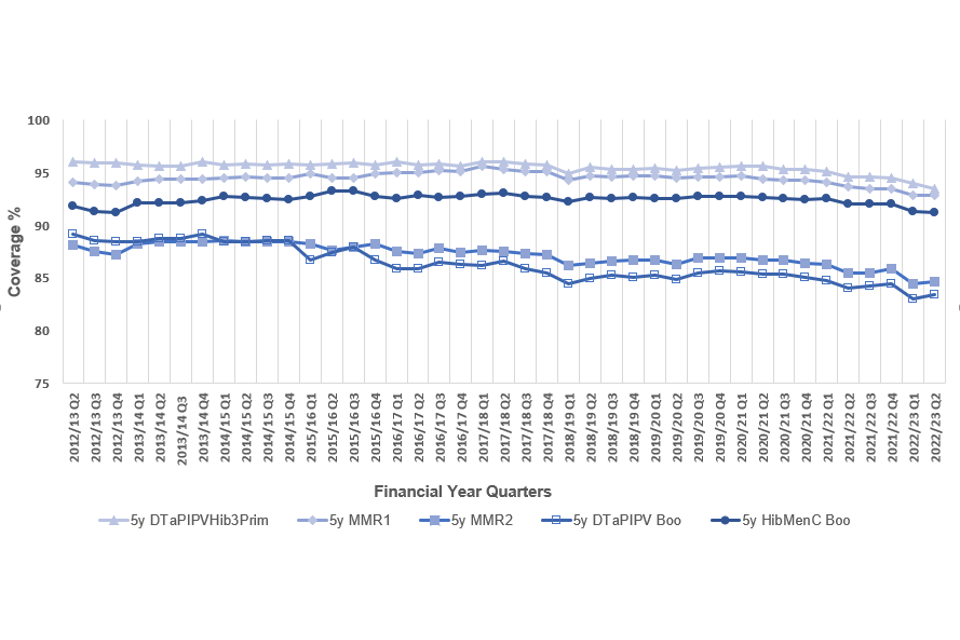
3.4 Neonatal hepatitis B vaccine coverage: England
National coverage at 12 months for 5 doses of a HepB-containing vaccine decreased from 91% to 89% compared with the previous quarter. Coverage of 6 doses of a HepB-containing vaccine reported for children who reached 2 years of age in the quarter (those born between July and September 2020) decreased 2% to 83% compared with the last quarter (85%) see separate data file accompanying this report.
The quality of neonatal HepB vaccine data is variable and coverage by former local teams can be based on small numbers. As such, data should be interpreted with caution. Where an area reported no vaccinated children, a check was made to ensure that this was zero reporting rather than absence of available data.
3.5 Neonatal BCG vaccine coverage: England
This is the second report for which BCG coverage has been collected for all eligible children in England measured at 3 months. The data captures BCG coverage for children born 1 January to 31 March 2022; it was provided for 150 of 152 local authorities in England published in the data tables associated with this report. Coverage in England was 67.5%.
4. Participation and data quality
Data was received from all health boards (HBs) in Scotland, Northern Ireland, and Wales. In England, local teams (LTs) and child health record departments (CHRDs) provided data for all upper tier local authorities (LAs) and the associated general practices (GP).
All English data were collected through NHS Digital’s strategic data collection service (SDCS). Individual LA and GP data including numerators, denominators, coverage and relevant caveats where applicable are available in the data tables associated with this report. GP level data was censored when individual values were less than 5.
5. Links for country-specific data
- Quarterly England
- Annual England
- Northern Ireland
- Scotland
- Wales
- COVER submission and publication dates
- Information for immunisation practitioners and other health professionals
